
Paper: Pernica JM et al. Short-Course Antimicrobial Therapy for Pediatric Community-Acquired Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatr 2021. PMID: 33683325
Clinical Question: Is a 5-day course of high-dose amoxicillin noninferior to a 10-day course for treatment of CAP in children not requiring hospitalization?
What They Did:
- Short-Course Antimicrobial Therapy for Pediatric Respiratory Infections (SAFER)
- 2-center, parallel-group, noninferiority randomized clinical trial comparing five days of high dose Amoxicillin followed by a 5-day course of placebo (intervention group) vs. five days of high-dose amoxicillin followed by a different formulation of high-dose amoxicillin (control group)
- Performed in Canada
- Participants, caregivers, outcome assessors, and study investigators were blinded to treatment assignment
Noninferiority Trials:
Noninferiority trials [Covered here on REBEL EM] seek to determine if a potential experimental treatment is “not much worse” than a standard treatment up to a predetermined noninferiority threshold (7.5% in this paper). Therefore, a statistically noninferior experimental treatment may be inferior but not statistically so until it crosses the threshold. The threshold is set by investigators and requires balancing between the potential benefits and harms of the standard treatment compared to the potential benefits and harms of the experimental treatment.
Outcomes:
- Primary Outcome: Clinical cure at 14–21 d follow up visit defined by:
- Improvement in first four days after enrollment, including defervescence
- Improvement in dyspnea, no tachypnea
- No more than one fever spike from day 4 to follow up visit
- Lack of requirement for additional antimicrobials or admission to the hospital because of persistent or progressive lower respiratory illness before follow up visit
- Secondary Outcomes:
- Clinical Cure not requiring additional intervention
- Improvement during the first four days after enrollment
- Lack of additional antibiotics or admission to hospital
- Strict PP analysis
- >80% of antimicrobial doses taken and no additional antibiotics taken for pneumonia infections
- Radiology confirmed pneumonia
- Number of days absent from school
- Number of days caregiver work disrupted
- Number of days of mild adverse reactions
- Incidence of serious reactions
- Participant adherence
- Recurrence of bacterial respiratory illness in the following month
- Clinical Cure not requiring additional intervention
Inclusion:
- Children 6 months to 10 years of age with CAP
- Well enough to be treated as outpatients
- CAP defined
- Fever (axillary temp > 37.5 ℃, oral temp > 37.7 ℃, rectal 38 ℃) in 48 hours before presentation
- Tachypnea, increased work of breathing, or auscultatory findings consistent with CAP
- Chest Xray findings consistent with CAP per ED physician
- Primary diagnosis of CAP per ED physician
Exclusion:
- Atypical pneumonia (empyema or necrotizing pneumonia)
- Preexisting pulmonary disease
- Congenital heart disease
- History of aspiration
- Malignant neoplasm
- Immunodeficiency
- Kidney disfunction
- Children who received > 24 hours of β-lactam therapy at presentation
- Children who received or > 5-day course of β-lactam therapy < 72 hours before presentation
- Children who received IV cephalosporin or azithromycin in the ED
- Children on warfarin or tetracyclines
- Suspicion of infectious mononucleosis
- > 48 h hospital stay in prior two months
- Lung abscess in prior six months
- CAP in the previous month
- Penicillin allergy
Results:
- 5406 patients diagnosed with pneumonia during the study
- 1910 were excluded
- 3215 missed
- 281 participants recruited
- 29 patients lost to follow up
- 252 had outcomes documented
- 100 patients (71.4%) in the 5-day amoxicillin group had radiologist confirmed pneumonia
- 108 patients (76.6%) in the 10-day amoxicillin group had radiologist confirmed pneumonia
- 63.5% of children swabbed in the 5-day amoxicillin group were positive for at least one virus (not all were tested)
- 61.9% of children swabbed in the 10-day amoxicillin group were positive for at least one virus (not all were tested)
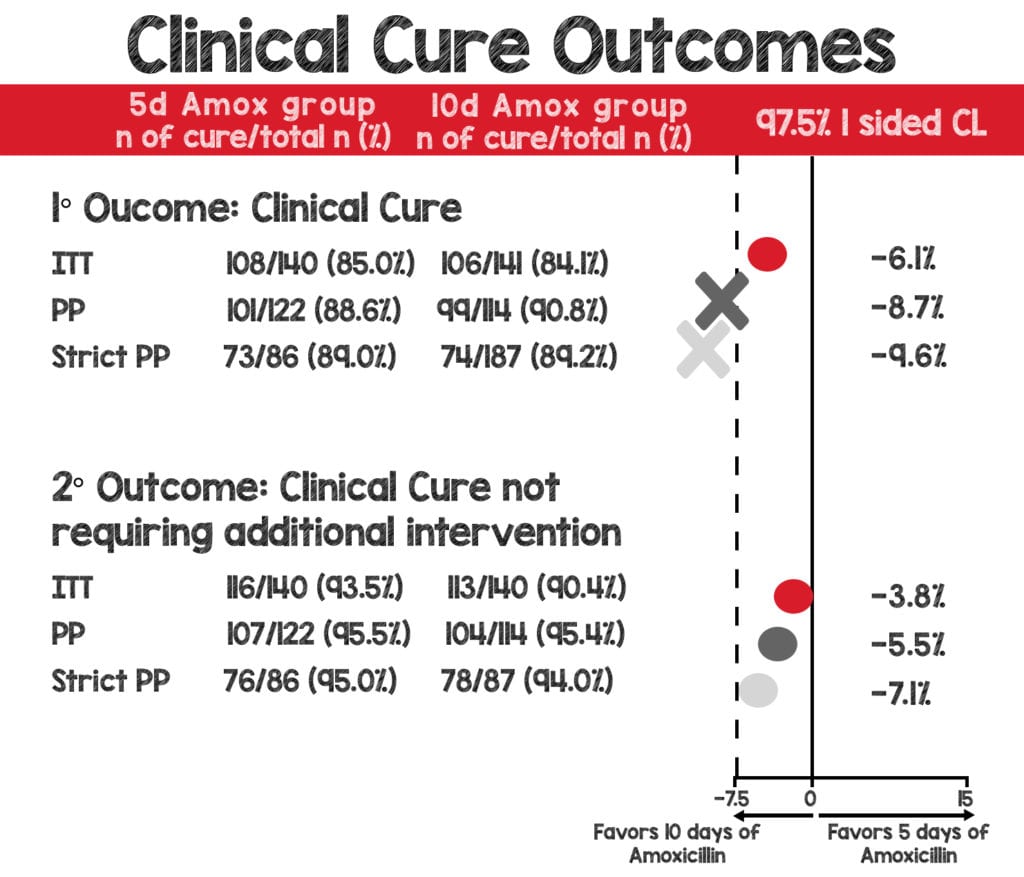
- Primary Outcome: Clinical cure at 14-21 days after enrollment
- ITT analysis: NONINFERIOR
- 108/126 (85.7%) in the 5-day amoxicillin group
- 106/126 (84.1%) in the 10-day amoxicillin group
- RD: 0.023; 97.5% CL: –0.016
- PP Analysis: CROSSED NONINFERIORITY THRESHOLD
- 101/114 (88.6%) in the 5-day amoxicillin group
- 99/109 (90.8%) in the 10-day amoxicillin group
- RD: –0.016; 97.5% CL: –0.087
- Strict PP Analysis: CROSSED NONINFERIORITY THRESHOLD
- 73/82 (89.0%) in the 5-day amoxicillin group
- 74/83 (89.1%) in the 10-day amoxicillin group
- RD: –0.011; 97.5% CL: –0.096
- ITT analysis: NONINFERIOR
- Secondary Outcome: Clinical Cure not requiring additional intervention
- ITT analysis: NONINFERIOR
- 116/124 (93.5%) in the 5-day amoxicillin group
- 113/125 (90.4%) n the 10-day amoxicillin group
- RD: 0.028; 97.5% CL: –0.038
- PP Analysis: NONINFERIOR
- 107/112 (95.5%) in the 5-day amoxicillin group
- 104/109 (95.4%) in the 10-day amoxicillin group
- RD: –0.006; 97.5% CL: –0.055
- Strict PP Analysis: NONINFERIOR
- 76/80 (95.0%) in the 5-day amoxicillin group
- 78/83 (94.0%) in the 10-day amoxicillin group
- RD: –0.004; 97.5% CL: –0.071
- ITT analysis: NONINFERIOR
Strengths:
- Attempts to tackle a relevant clinical question—reducing antibiotic usage in children diagnosed with CAP who did not require hospitalization
- Multicenter, double-blind, randomized study design increases internal validity
- Primary and secondary outcomes were patient-oriented
- Inclusion and exclusion criteria applied to real-world clinical practice
- Listed patient characteristics were similar in both groups
- Sound randomization and blinding protocol
- High adherence to protocol in both groups
- Performed intention-to-treat and per-protocol analysis
- Pragmatic ED study translates to clinical practice
- The appearance and taste for the placebo and the amoxicillin for the second 5 days were similar to each other and different than the amoxicillin used for the 1st 5 days of treatment to preserve blinding in both groups
Limitations:
- Single country Canadian study where pathogen and antimicrobial resistance rates may differ from individual practice
- A small trial of 281 patients, 29 of which were lost to follow up
- Likely a convenience sample > 3200 patients were missed for potential enrollment adds to selection bias
- >60% of children swabbed in both groups were positive for at least one virus
- The measures of improvement are subjective
- They did not achieve the 95% cure rate they sought, and therefore the study is underpowered
- “Clinical cure not requiring additional intervention” (secondary outcome) was added after subgroup analysis
- Could not definitively establish bacterial pneumonia
- Laboratory testing was optional
Discussion
- The study appropriately uses the noninferiority design.
- The new treatment approach being investigated shortens the treatment course, which would cost less to the patient, have a lower side effect profile, and on a global scale potentially lower antimicrobial resistance.
- It seems somewhat like a comparison of five days of antibiotics to ten days of antibiotics in children with viral pneumonia.
- The high rate of viral pathogens (>60% of pts who were swabbed) detected is congruent with other large studies on pediatric patients hospitalized with pneumonia. (Jain et al. 2015)
- It bears mentioning that other studies have found bacterial and viral coinfection rates as low as 7%. (Jain et al. 2015)
- Does the duration of antibiotic therapy matter in a group of patients where the majority would not benefit from antibiotics?
- Simply put, would these patients have improved with ten days, five days, or zero days of antibiotics.
- The study did not achieve the 95% cure rate it estimated for the primary outcome.
- Actual cure rates varied from 85%–90% for primary outcomes.
- If the experimental therapy is truly noninferior to the standard therapy, then a sample size from 500–700 patients would be needed based on the cure rate achieved.
- The authors provided an analysis of Intention-to-treat and Per Protocol.
- ITT analysis represents children randomized to a particular therapy, whether they were adherent or not.
- ITT is more reflective of real-world experience.
- Per Protocol analysis represents children randomized to a particular therapy and who were adherent to the study protocol.
- For the primary outcome, ITT was statistically noninferior, but Per Protocol crossed the preset noninferiority threshold of 7.5%.
- The 5-day amoxicillin group represents more adherent patients than the 10-day amoxicillin group (90.4% in 5d vs. 86.4% in 10d).
- Additionally, 76.6% of the 10-d amoxicillin group had radiologist confirmed pneumonia, while only 71.4% of the 5-d amoxicillin group had radiologist confirmed pneumonia.
- So the 10-d amoxicillin group potentially had higher rates of pneumonia and were less adherent to antibiotics.
- Underestimates the benefit of the standard therapy leading to a potentially false conclusion of noninferiority
- Per Protocol analysis and strict Per Protocol analysis crossed the noninferiority threshold
- Children in these groups were more adherent to therapy.
- If 5 days of amoxicillin is “no worse” than 10 days then PP analysis should not cross the threshold.
- ITT analysis represents children randomized to a particular therapy, whether they were adherent or not.
- The investigators did find that short-course treatment was statistically noninferior in PP, ITT, and strict PP with less stringent clinical cure definitions determined on subgroup analysis post hoc.
- Clinical cure not requiring additional intervention does seem pragmatic.
- Many children may have been documented as a treatment failure who likely would not have been outside the clinical trial.
- However, the sample sizes were underpowered.
- Is this data dredging?
- REBEL EM supports shorter courses of antibiotics when clinically appropriate.
- However, this paper does not provide enough evidence to support practice change.
- More extensive trials are still needed.
Authors Conclusion: “Short-course antibiotic therapy appeared to be comparable to standard care for the treatment of previously healthy children with CAP not requiring hospitalization. Clinical practice guidelines should consider recommending 5 days of amoxicillin for pediatric pneumonia management in accordance with antimicrobial stewardship principles.”
Clinical Bottom Line: The investigators found that short-course antibiotic therapy was noninferior to standard care in healthy children with CAP not requiring hospitalization. However, many children had viral pathogens and may have improved despite antibiotics and not because of them. Additionally, the study was underpowered, and statistically significant noninferiority in secondary outcomes resulted from subgroup analysis determined post hoc. Thus, this paper does not provide enough evidence to support changing clinical practice.
References:
- Propersi M. The Pneumonia Short Treatment Trial: Antibiotic Treatment for 3 days vs 8 days. REBEL EM blog, May 3, 2021. Available at: https://rebelem.com/the-pneumonia-short-treatment-trial-antibiotic-treatment-for-3-days-vs-8-days/. Published 2021. Accessed June 8, 2021.
- Pernica JM, Harman S, Kam AJ, et al. Short-Course Antimicrobial Therapy for Pediatric Community-Acquired Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatr. 2021;175(5):475-482. [Link is here]
- Swaminathan A. Omadacycline, the NEJM and Non-Inferiority Studies. REBEL EM – Emergency Medicine Blog. Available at: https://rebelem.com/omadacycline-the-nejm-and-non-inferiority-studies/. Published 2019. Accessed June 8, 2021.
- Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. [Link is here]
For More Thoughts on This Topic Checkout:
The SGEM: SGEM#338 – Are Children With CAP Safe and Sound if Treated for 5D Rather Than 10D of Antibiotics?
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)



