Background Information:

Paper: Corral-Gudino L, et al; GLUCOCOVID investigators. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021 Feb 3, Epub ahead of print. PMID: 33534047
Clinical Question:
- What is the role of a 6-day course of methylprednisolone in patients with COVID-19 pneumonia at risk of developing respiratory failure and ARDS?
What They Did:
- Partially randomized, open-label, controlled, two-arm, parallel group, trial conducted at 5 hospitals in Spain between April and May 2020
- Preference Arm: If clinical team decided a strong preference for glucocorticoid therapy existed, patient was allocated to this arm and immediately placed in the experimental group below
- Randomized Arm: Patients were randomized in standard 1:1 fashion to one of the following two groups:
- Control group: Standard of care treatment
- Experimental group: Standard of care treatment PLUS 40 mg of Methylprednisolone IV every 12 hours for 3 days and then 20 mg every 12 hours for 3 days
- Standard of care therapy included the following: Symptomatic treatment with acetaminophen, oxygen therapy, thrombosis prophylaxis with low-molecular weight heparin and antibiotics for co-infection
- In both groups, Azithromycin, Hydroxychloroquine, Lopinavir plus Ritonavir, IL-blocking agents and other therapies were frequently prescribed as indicated
- This is the pre-planned interim analysis of the first 90 patients enrolled
Inclusion Criteria:
- Hospitalized patients over 18 years of age with a laboratory confirmed SARS-CoV2 infection
- Additional criteria included the following:
- Symptom duration of at least 7 days
- Radiologic evidence of lung disease via chest x-ray or CT-scan
- Moderate to severe disease with abnormal gas exchange: PaO2/FiO2 < 300 or SaO2/FiO2 <400, or at least 2 criteria of the BRESCIA-COVID Respiratory Severity Scale (BCRSS)
- Laboratory markers of inflammation:
- C-Reactive Protein > 15 mg/dL
- D-dimer > 800 mg/dL
- Ferritin > 1000 mg/dL
- IL-6 Level > 20 pg/mL
Exclusion Criteria:
- Intubated or mechanically ventilated patients
- Those hospitalized in the ICU
- Already treated with corticosteroids or immunosuppressive therapy at time of enrollment
- Chronic kidney disease on dialysis
- Pregnant patients
- Those who refused to participate
Outcomes:
Primary
- Composite endpoint which included the following:
- In-hospital all-cause mortality
- Escalation to ICU admission
- Progression of respiratory insufficiency that required non-invasive ventilation
Secondary
- The effects of the individual components of the composite endpoints
- Laboratory biomarkers at baseline and 6 days after inclusion
Results:
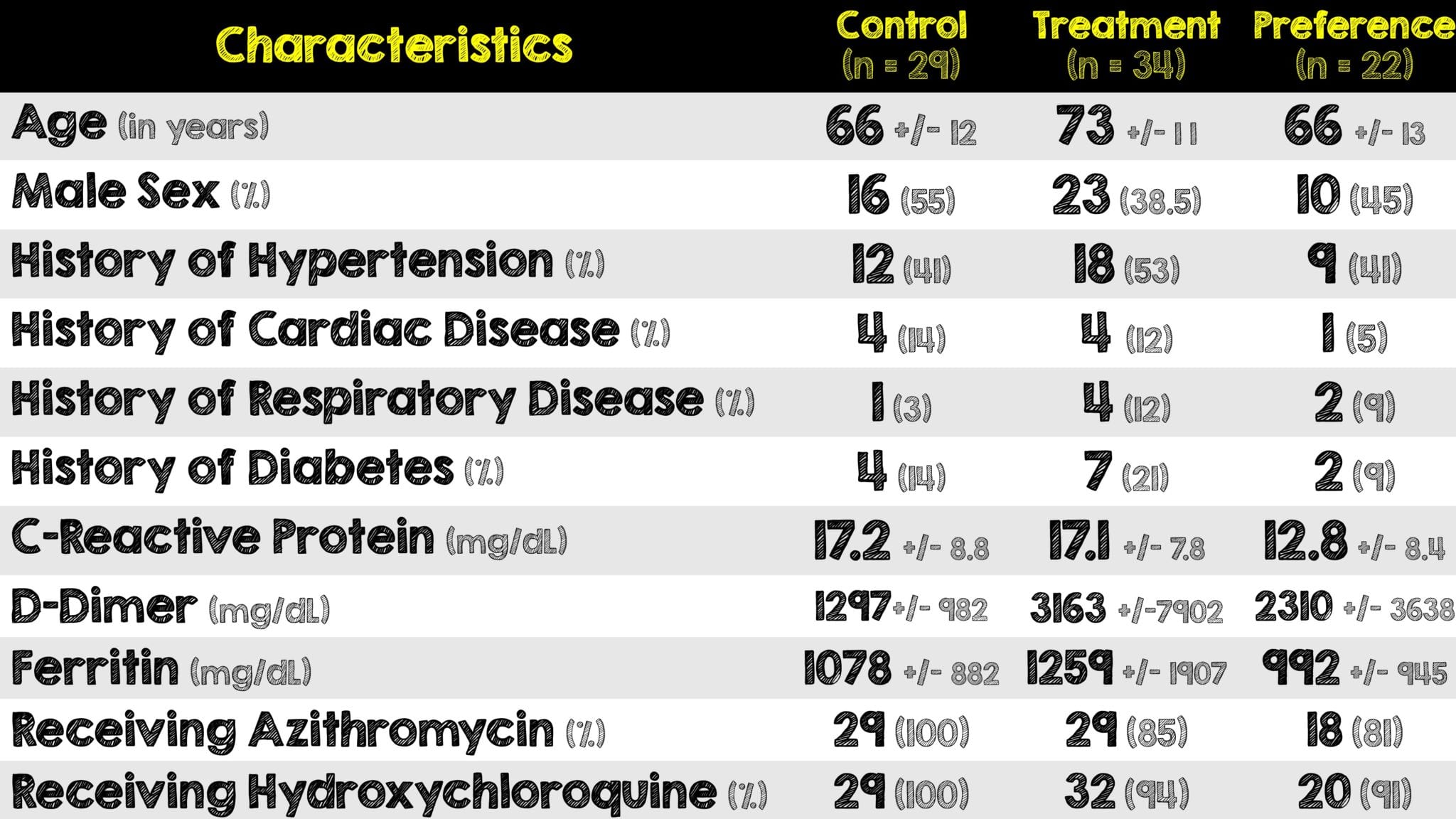
- 90 patients were initially enrolled and 5 met exclusion criteria
- Of the 85 remaining, 22 were preferentially given methylprednisolone and the remaining 63 were randomized
Critical Results:

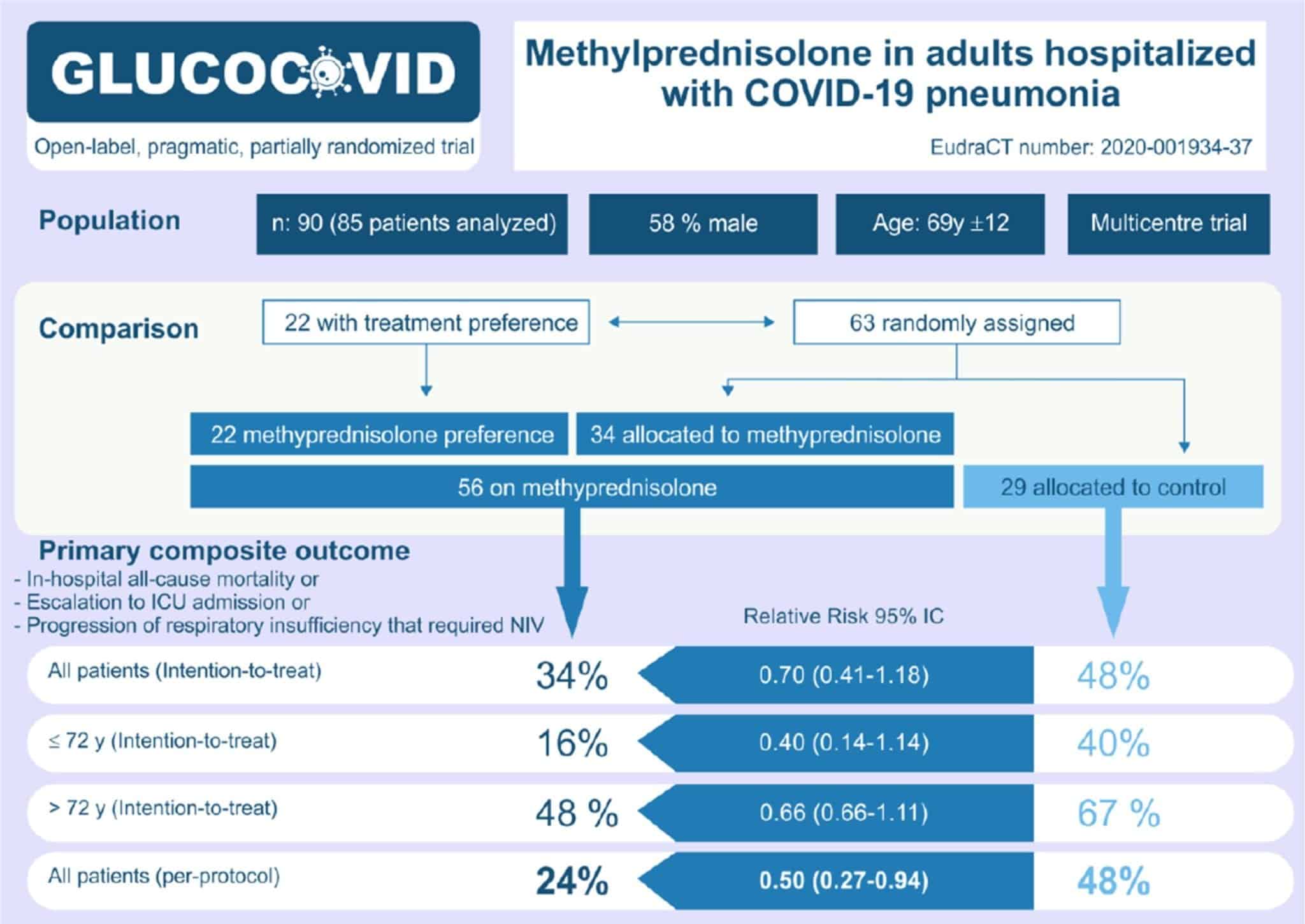
(Image from original paper)
- Although more patients in the methylprednisolone group received Tocilizumab (IL-6 Blocker) and Anakinra (IL-1 Blocker), the results were similar to the whole group once these patients were excluded from the analysis
- The following two analyses were performed to assess the effect of age on the primary outcome:
- Unadjusted: Showed no statistically significant difference
- Age-stratified: Methylprednisolone was associated with a significantly lower risk of bad outcome
- No major side effects occurred except for an expected hyperglycemia in 12 patients (21%) in the methylprednisolone group that was not statistically significant (p = 0.006)
- The primary driver of the primary compose outcome was ICU admissions, which in an unblinded trial, is a subjective outcome
Strengths:
- Attempts to answer a clinically relevant question in a very specific patient population using a widely available medication
- Inclusion of the clinician preferential arm helps to avoid inclusion bias
- In addition to laboratory confirmed positive COVID-19 test, they had very specific and well thought out inclusion criteria
- Utilized nationally developed protocols for COVID-19 standard of care
- Performed both an intention-to-treat analysis to include all patients who took at least one dose of methylprednisolone and a per-protocol analysis which excluded 7 patients who received less than 3 doses (due to clinical deterioration and escalation to ICU level of care)
Limitations:
- Small sample size as it is a pre-planned interim analysis and thus making this trial not powered to treatment with individual endpoints
- Complicated trial design with the preferential arm. This served to imbalance baseline characteristics of both groups such as older patients being in the treatment arm
- Did not specify if patients were identified via consecutive vs convenient enrollment, and thus there may be an inherent sampling bias
- Composite primary outcome is composed of individual components that are not of equal severity. Mortality is not the same as escalation to the ICU or requiring NIV
- Practice variability and differences in patient care exists across the 5 different hospitals and more importantly, all 5 were in the same country thus limiting external validity
- Although intubated and mechanically ventilated patients were excluded, it’s unclear exactly how many patients were actually on oxygen therapy
- Lack of follow-up for adverse reactions, readmissions, etc
Discussion:
- This is a very specific patient population (those not sick enough for the ICU but admitted with moderate to severe disease) and the authors are applauded for attempting to isolate the effect of a relatively inexpensive, widely available and easy to administer medication
- The authors were looking for a 50% reduction in the primary composite outcome, which is very large. This trial is grossly underpowered to achieve this.
- Although the age of patients in the experimental group was higher, the authors performed two additional analyses to account for this and confirmed that advanced age is a risk factor for poor outcome. This confounding variable serves to be clinically relevant and supports already existing knowledge that older patients are unlikely to survive following exposure to COVID-19.
- Several patients in this study received glucocorticoids but deteriorated quickly and had their care escalated to the ICU. This raises questions around the utility and benefit of treating COVID-19 patients with glucocorticoids earlier vs the risk of early immunosuppression while still in the viral phase of the illness
- The lack of side effects (except for hyperglycemia) with administration of methylprednisolone is reassuring. The lack of harm or adverse effects is in contrast to the RECOVERY Trial using Dexamethasone, (already covered here on REBEL EM), which expectedly has more long term adverse effects
- Confounding variables of age and baseline respiratory status aside, both groups of patients received a LOT of medications: Azithromycin, Hydroxychloroquine, Acetaminophen, etc. It’s difficult, if not impossible, to ascertain whether any of these had any kind of synergistic benefit with methylprednisolone specifically against COVID-19
- The exact number of patients requiring oxygen therapy (across both groups) was not reported. This limits any conclusions from being drawn regarding the benefit of methylprednisolone in patients requiring ANY oxygen therapy at all
- It’s important to keep in mind that this was a pre-planned interim analysis and not powered to explore the association of treatment with individual endpoints
Author’s Conclusions:
- “A short course of methylprednisolone had a beneficial effect on the clinical outcome of severe COVID-19 pneumonia, decreasing the risk of the composite end point of admission to ICU, NIV or death.”
Our Conclusion:
- The notion of early steroid administration causing worsening disease due to immunosuppression in the viral stage of the illness continues to remain controversial. Small studies such as this are not powered well enough to give us useful information, however it does show trends towards improvement.
Clinical Bottom Line:
- Although a pre-planned interim analysis from a small study, the use of methylprednisolone in hospitalized COVID-19 patients, not sick enough for the ICU but admitted with moderate to severe disease, there was a benefit in the primary composite outcome. This however was driven by ICU admission, which is a subjective outcome in an unblinded trial.
REFERENCES:
- Corral-Gudino L, et al; GLUCOCOVID investigators. Methylprednisolone in adults hospitalized with COVID-19 pneumonia : An open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021 Feb 3, Epub ahead of print. PMID: 33534047
- Wu C, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul; PMID: 32167524
- Fadel R, et al. Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19. Clin Infect Dis. 2020 Nov; PMID: 32427279
- Wang D, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar; PMID: 32031570
- RECOVERY Collaborative Group, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021 Feb; PMID: 32678530
- Rezaie S, “The RECOVERY Trial: Dexamethasone for COVID-19?”, REBEL EM blog, June 23, 2020. Available here
For More Thoughts on This Topic Checkout:
- First 10 in EM: Steroids for COVID-19
- REBEL EM: It’s Raining Steroids in COVID-19
- CriticalCareNow: Corticosteroids in COVID-19: The Pendulum Swings Back
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @Srrezaie)




