REBEL Cast Ep123: Reduced-Dose Systemic Peripheral Alteplase in Massive PE?
Paper: Aykan AC et al. Reduced-Dose Systemic Fibrinolysis in Massive Pulmonary Embolism: A Pilot Study. Clin Exp Emerg Med 2023. PMID: 37188358
Clinical Question: What is the efficacy and safety of low-dose (25mg) prolonged administration (over 6hrs) of alteplase in patients with massive PE?
What They Did:
- Single-center, pilot prospective observational cohort trial in Turkey
- Thrombolysis
- 25mg of alteplase without a bolus was administered over 6 hours by peripheral IV infusion
- If hemodynamic instability persisted despite first dose of thrombolysis, a second 6hr infusion of 25mg alteplase without bolus was administered (No patients in the study required this)
- Did not use concomitant heparin anticoagulation with thrombolysis
- Heparin was administered as a 70U/kg bolus followed by a 1000U/h infusion with a target activated PTT between 1.5 and 2.5x the control started immediately after infusion of thrombolysis completed
- Patients were all converted to warfarin for discharge
- TTE
- All patients underwent TTE before thrombolysis, within an hour after thrombolysis, before discharge (5 to 7d) and a month after thrombolysis
- PASP estimated from tricuspid valve regurgitant jet velocity
- Maximum dimension of RA/LA measured in 4-chamber view
- Diameter and collapsibility of IVC noted
- Pulmonary HTN defined as PASP >40mmHg
- RV enlargement defined as RV/LV ratio >0.9
- Tricuspid Annular Plane Systolic Excursion (TAPSE) also recorded
- Tissue Doppler Derived Tricuspid Annular Systolic Velocity recorded
- Tei-Myocardial Performance Index (MPI/Tei) recorded
- CT
- All patients underwent 64 slice CTPA for definitive diagnosis of PE at admission
- Additional CTPA 24 hours after completion of thrombolysis if eGFR >60mL/min/1.73m2
- Criteria for Thrombolytic Success Included:
- Doppler documentation of resolution of increased PASP (<40mmHg)
- Decreased RV diameter (at least 25% decrease of RV/LV diameter)
- Restoration of RV function (TAPSE>16mm)
- Systolic Wave Prime (S’) >10.0cm/s
- Tissue Doppler Derived RV MPI > 0.55
- Clinical improvement of symptoms and restoration of stable hemodynamic status immediately after thrombolysis
- Complete success = Clinical improvement of symptoms and restoration of a stable hemodynamic status along with at least 3 other criteria without resultant death and nonfatal major complications
Outcomes:
- Primary:
- In-hospital mortality
- Major complications
- Ischemic stroke, ICH, embolism (coronary or peripheral), bleeding requiring transfusion
- Pulmonary HTN while in hospital
- RV dysfunction while in hospital
- Secondary:
- 6 month mortality
- Development of pulmonary hypertension at 6 months
- RV dysfunction at 6 months
Inclusion:
- Adult patients (≥18 years of age)
- Confirmed massive PE
- Massive PE Definition
- Acute PE with sustained hypotension (SBP <90mmHg for at least 15 minutes or requiring inotropic support, not due to a cause other than PE, such as arrhythmia, hypovolemia, sepsis, or LV dysfunction)
- Pulselessness
- Persistent profound bradycardia (HR<40BPM with signs or symptoms of shock)
- Massive PE Definition
Exclusion:
- Prior ICH
- Known structural intracranial cerebrovascular disease (i.e. AV malformation)
- Known malignant intracranial neoplasm
- Ischemic stroke within 3 months
- Suspected aortic dissection
- Active bleeding or bleeding diathesis
- Recent surgery encroaching on the spinal canal or brain
- Recent significant closed-head or facial trauma with radiographic evidence of bony fracture or brain injury
Results:
- 37 consecutive patients with massive PE were enrolled
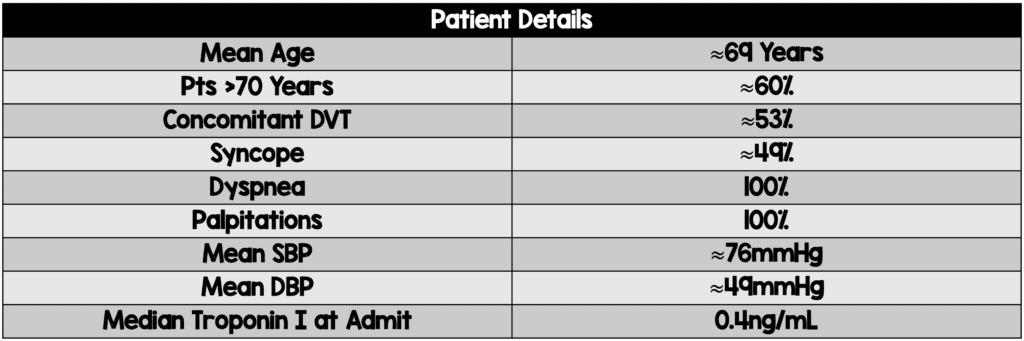
- Blood pressure recovered within a few hours after initiation of thrombolysis in all cases
- Mortality
- Primary Efficacy Outcome: 1 in-hospital death (3.1% in the paper but 2.7% in table 2) on day 6 of hospitalization due to malignant ventricular arrhythmia (Pt had ischemic dilated cardiomyopathy with LVEF of 20% and acute on chronic renal failure)
- 2 additional deaths within 6 months (Both pts had malignancy)
- Not implicitly stated whether these deaths are attributed to PE, however it doesn’t sound like they are based on the patient descriptions
- TTE Results from Admission to Post Thrombolysis:
- Primary Efficacy Outcome: Mean PASP ≈57mmHg to 34mmHg (p<0.001)
- Mean PASP continued to decrease prior to discharge ≈34mmHg to ≈30mmHg (p<0.001)
- Mean PASP preserved at 6 month follow up ≈29mmHg
- No patients with pulmonary hypertension at 6 months
- Primary Efficacy Outcome: Mean TAPSE ≈1.43cm to 2.07cm (p<0.001)
- RV/LV Diameter ≈1.37 to ≈0.99 (p<0.001)
- Mean MPI/Tei Index≈ 0.47 to ≈0.55 (p<0.001)
- Systolic Wave Prime (S’) ≈10 to ≈15
- Primary Efficacy Outcome: Mean PASP ≈57mmHg to 34mmHg (p<0.001)
- Follow Up CTPA 24hrs After Thrombolysis
- Out of 37 patients, 18 (56.3%) underwent repeat CTPA 24hrs after thrombolysis
- Out of these 18 pts total lysis of thrombus was observed in 16pts (88.9%) and the remaining 2pts had >75% lysis of thrombus
- Complications Post Thrombolysis:
- Primary Safety Outcome: No major bleeding or stroke observed
- 3 patients with minor bleeding
- 2pts with epistaxis (2 days after thrombolysis)
- 1pt with gingival bleeding (3 days after thrombolysis)
- All bleeding events occurred during heparin infusion and stopped with gentle compression without recurrence
- 6pts (17.6%) had major bleeding and 2pts (5.68%) had minor bleeding due to warfarin
- Out of 37 patients, 18 (56.3%) underwent repeat CTPA 24hrs after thrombolysis
Strengths:
- Consecutive patients enrolled which minimizes selection bias
- All patients followed for 6 months (No loss to follow up)
Limitations:
- Single center, nonrandomized observational trial
- No comparison group receiving standard therapy (i.e. compared to half-dose 50mg or full dose 100mg of alteplase)
- Small sample size with few complications makes this an underpowered study to make any firm conclusions about bleeding risks
- Lots of missing methodology
- Unclear what time period patients were recruited
- How strict were authors in consecutive recruitment (? selection bias)
- Unclear therapies prior to lytics (i.e. Pressors, heparin, etc)
- Who performed echos and unclear degree of consistency (Echo has some subjectivity to it)
Discussion:
- PE is a Spectrum of Disease
- Massive PE is also a spectrum of disease
- This study doesn’t appear to include critically ill massive PE patients who are peri-arrest
- Dripping alteplase over 6 hours is most likely not going to be the appropriate therapy in the more severe massive PE patients
- Primary Efficacy Outcome
- Multiple primary efficacy outcomes were listed in this study, which can be problematic when you get multiple outcomes of varying statistical significance. In this trial all the primary efficacy outcomes were statistically significant, and the authors clearly defined what they meant by complete success in this trial
- Complications While in Hospital
- No cases of major bleeding or ICH in this study
- The 3 patients who had minor bleeding at 48 to 72hrs were most likely due to the heparin infusion and not associated with thrombolysis
- The cohort is simply too small with not enough complications for the study to be powered correctly for this outcome
- IV Access
- These authors gave alteplase through a peripheral IV which we do in stroke patients, but that is typically done over 1hr not over 6hrs
- If I was going to do this (25mg alteplase over 6hrs) I would want a central venous catheter to avoid the potential of infiltration or if the patient decompensates further and needs central venous access after the fact there is a higher risk of hematoma/bleeding
- Also, I would already have an arterial line in place before starting thrombolysis for continuous hemodynamic monitoring
- Heparin Dosing
- Heparin was administered as a 70U/kg bolus followed by a 1000U/h infusion with a target activated PTT between 1.5 and 2.5x the control started immediately after infusion of thrombolysis completed
- Prior studies have found marked increase in bleeding when lytics and heparin are given together
- After thrombolysis I typically don’t bolus heparin and just start the infusion
- Also I don’t start the heparin infusion immediately after thrombolysis, I wait for the PTT to be <2x the control before starting
- A dose of 0.5 to 4.0mg/hr typically given in EKOS therapy (See Below). This trial gave 25mg over 6hrs (≈4mg/hr)
- ULTIMA Trial [5]:
- 59pts with massive/submassive PE
- Used alteplase at a dose of 1mg/hr x5hrs, then 0.5mg/hr x10hrs
- Max Dose ≈20mg
- No major bleeding
- SEATTLE II Trial [6]:
- 150pts with massive/submassive PE
- Used alteplase at a dose of 1mg/hr x24hrs
- Max Dose ≈25mg
- 1 severe/life-threatening hemorrhage (Groin hematoma requiring vasopressor support)
- No ICH
- OPTALYSE-PE Trial [7]:
- 101pts with submassive PE
- Used alteplase at a dose of 8mg/2hrs (4mg/hr), 8mg/4hrs (2mg/hr), 12mg/6hrs (2mg/hr), and 24mg/6hrs (4mg/hr)
- Max Dose 24mg
- No major bleeding with 8mg/2hrs, 8mg/4hrs, and 12mg/6hrs
- 2 major bleeding episodes occurred in the 24mg/6hr group
- The max dose any patients got was ≈25mg (Max Dose Range ≈20mg to ≈25mg). Major bleeding seemed to occur in the drips that ran for over 15hrs (3 pts out of 310 [≈1%]); But no cases of major bleeding for drips ≤15hrs
- To take this one step further this trial raises the question of whether EKOS even necessary or is it an overly expensive intervention that potentially increases complications without improving outcomes?
- ULTIMA Trial [5]:
- Although this was not a randomized clinical trial and there was no comparator arm we do have two trials on submassive PE with half-dose alteplase (50mg) given over 2hours [8] and half-dose alteplase (50mg) given in Massive PE [3]
- MOPETT Trial [8]
- 121pts with submassive PE (Called “moderate PE” in the study)
- Randomized to half dose thrombolysis vs anticoagulation alone
- For patients weighing ≥50kg a total dose of 50mg given (10mg bolus by IV push followed by 40mg infusion over 2hrs)
- For patients weighing <50kg a total dose of 0.5mg/kg given (10mg bolus by IV push followed by the remainder over 2hrs)
- Primary endpoints consisted of pulmonary HTN and composite of pulmonary HTN and recurrent PE at 28mos
- Pulmonary HTN at 28mos: 16% half dose thrombolysis vs 63% anticoagulation alone
- Composite Pulmonary HTN and Recurrent PE at 28mos: 16% half dose thrombolysis vs 63% anticoagulation alone
- There were 0 cases of bleeding in either arm
- PEAPETT Trial [3]
- 23 patients with PEA cardiac arrest due to confirmed massive PE
- All pts received 50mg of alteplase as an IV push while CPR was ongoing
- ROSC occurred in 2 to 15 min after alteplase administration in all but one patient
- There was no minor or major bleeding despite chest compressions
- What this current trial [9] is really adding to the literature is an even lower dose of thrombolysis (25mg) efficacious?
- We already know from the MOPETT trial [8] and PEAPETT trial [3] that half-dose alteplase (50mg) had zero cases of bleeding so this current trial just tells us what we already know. 25mg of alteplase has less risk of bleeding than 50mg of alteplase
- Additionally, if 25mg can treat massive PEs [9], this could also be extrapolated to less severe high-risk submassive PEs (Although I would still love to see a head-to-head trial of 25mg vs 50mg)
- MOPETT Trial [8]
Author Conclusion: “Results of this pilot study suggest that low-dose prolonged infusion of tPA is an effective and safe therapy in patients with massive PE. This protocol was also effective in decreasing PASP and restoration of RV function.”
Clinical Take Home Point: Low dose (25mg) alteplase given as a prolonged infusion (over 6hrs) is a promising effective and safe therapy in patients with massive PE and provides an alternative to full dose (100mg) and half-dose (50mg) alteplase. Larger RCTs comparing doses of alteplase are warranted to confirm these findings.
References:
- Jaff MR et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension: A Scientific Statement from the American Heart Association. Circ 2011. PMID: 21422387
- Wan S et al. Thrombolysis Compared with Heparin for the Initial Treatment of Pulmonary Embolism: A Meta-Analysis of the Randomized Controlled Trials. Circ 2004. PMID: 15262836
- Sharifi M et al. Pulseless Electrical Activity in Pulmonary Embolism Treated with Thrombolysis (from the “PEAPETT” Study). AJEM 2016. PMID: 27422214
- Wang C et al. Efficacy and Safety of Low Dose Recombinant Tissue-Type Plasminogen Activator for the Treatment of Acute Pulmonary Thromboemolism: A Randomized, Multicenter Controlled Trial. CHEST 2010. PMID: 19741062
- Kucher N et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circ 2014. PMID: 24226805
- Piazza G et al. A prospective, Single-Arm Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACCC Cardiovasc Interv 2015. PMID: 26315743
- Tapson VF et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Reisk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv 2018. PMID: 30025734
- Sharifi M et al. Moderate Pulmonary Embolism Treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013. PMID: 23102885
- Aykan AC et al. Reduced-Dose Systemic Fibrinolysis in Massive Pulmonary Embolism: A Pilot Study. Clin Exp Emerg Med 2023. PMID: 37188358
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (X: @EMSwami)



