REBEL Cast Episode 76 – The 65 Trial – Shooting for a Lower MAP in Older Patients with Sepsis?
Paper:
- Lamontagne F et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients with Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA 2020. PMID: 32049269
Clinical Question:
- Does reducing exposure to vasopressors through permissive hypotension (MAP target 60 – 65mmHg) reduce mortality at 90 days in ICU patients ≥65 years of age with vasodilatory hypotension?
What They Did:
- Pragmatic, open-label, multicenter, parallel group, randomized clinical trial
- 65 ICUS in the UK including 2600 randomized patients
- Patients randomized to:
- Permissive hypotension = MAP target 60 – 65mmHg
- Usual care (at the discretion of treating clinicians)
- Choice of vasopressor, as well as all other interventions were also at the discretion of the treating physician
Outcomes:
- Primary: All-cause mortality at 90d
- Secondary:
- Mortality at discharge from ICU
- Mortality at discharge from hospital
- Duration of survival to longest available follow-up
- Duration of advanced respiratory and renal support during ICU stay
- Days alive and free of advanced respiratory support and renal support within first 28d
- Duration of ICU and hospital LOS
- Cognitive decline (Assessed using the Informant Questionnaire on Cognitive Decline in the Elderly – IQCODE) in survivors at 90d and 1yr
- Health related quality of life (Assessed using the EuroQoL 5-dimension 5-level (EQ-5D-5L)) questionnaire in survivors at 90d and 1 year
- Adverse events up to ICU discharge
Inclusion:
- ≥65 years of age
- Vasodilatory hypotension (assessed by treating clinician)
- Randomized within 6hrs of commencing a vasopressor infusion (for at least 1hr)
- Adequate fluid resuscitation (as assessed by the treating clinician) completed or ongoing
- Vasopressors were expected to continue for ≥6hrs
Exclusion:
- Vasopressors being used solely as therapy for bleeding, acute ventricular failure (left or right) or post-cardiopulmonary bypass vasoplegia
- Ongoing treatment for brain injury or spinal cord injury (Contraindications to permissive hypotension)
- Death perceived as imminent
- Previous enrollment to the 65 trial
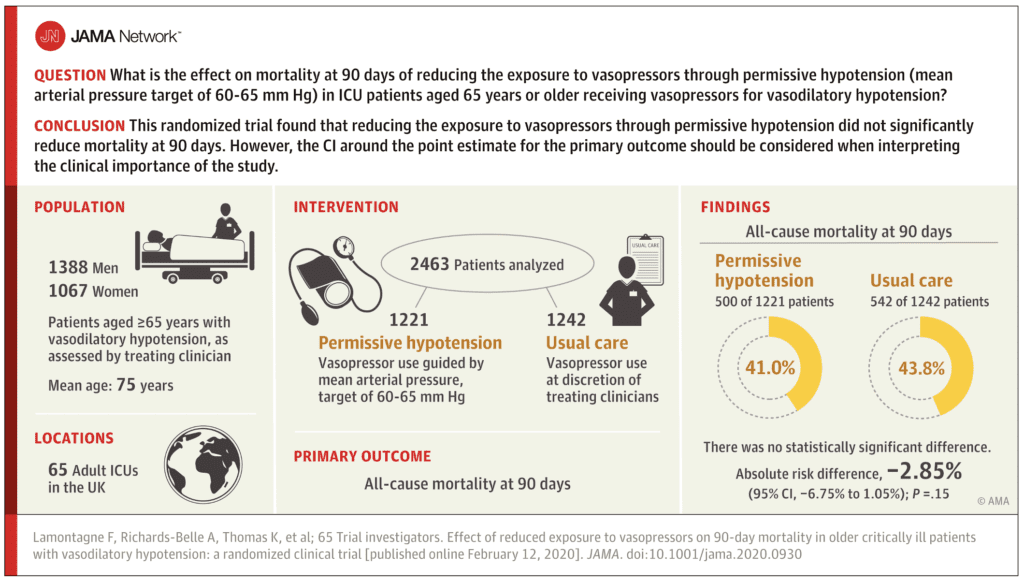
Results:
- 10,755 patients ≥65 years with vasodilatory hypotension and receiving vasopressors were screened
- 6,484 met inclusion criteria, but after applying exclusion criteria only 2930 were potentially eligible
- 2600 patients randomized
- 2463 patients (95%) included in the analysis of the primary outcome
- Mean age 75 years
- All-Cause 90d Mortality (Primary Outcome):
- Permissive Hypotension: 500/1221 (41.0%)
- Usual Care: 544/1242 (43.8%)
- Absolute Risk Difference = -2.85%; 95% CI -6.75 to 1.05; P = 0.15
- Unadjusted RR 0.93; 95% CI 0.85 – 1.03
- Adjusted RR 0.82; 95% CI 0.68 to 0.98
- Best- and worst-case sensitivity analyses did not alter the primary results
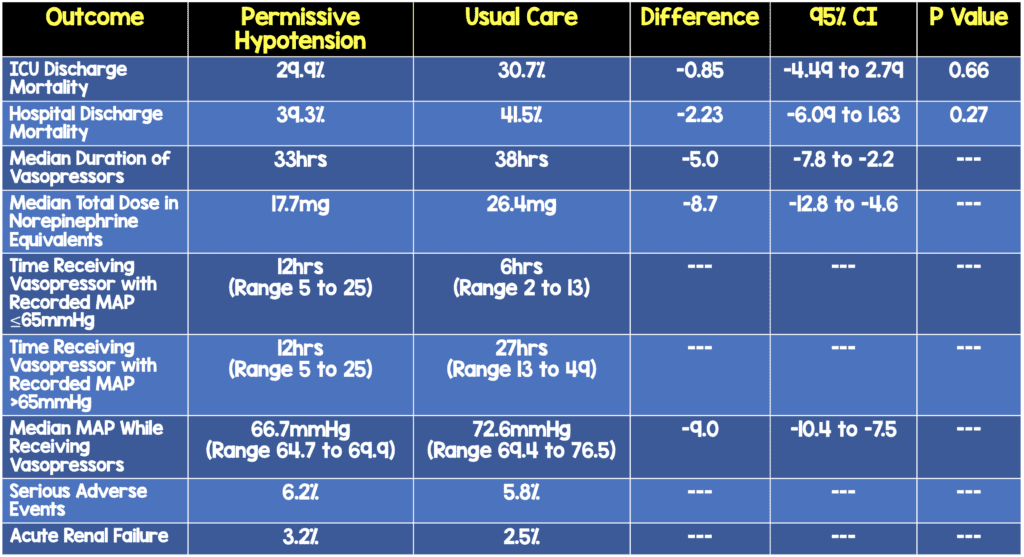
- No difference in need for advanced respiratory support, renal support, duration of ICU stay, cognitive decline, or quality of life
Strengths:
- Largest randomized clinical trial questioning the traditional practice of targeting a MAP 65mmHg in an older patient population (i.e. ≥65 years). Only 2 studies have been conducted to establish a minimally clinically important difference for critically ill patients ≥65 years with vasodilatory hypotension, both of which were subgroup analyses of larger studies [2][3]
- Monitored adherence to the protocol with objective definition of appropriate reduction in dose (or discontinuation) of vasopressors when MAP greater than target limit of 65mHg. Deviation was defined by failure to reduce (or discontinue vasopressors while the MAP remained higher than 65mmHg for 3 consecutive hours
- Performed in 65 ICUs increasing eternal validity of the results
- Targeted difference (60 – 65mmHg vs usual care) was present in the median difference in the MAP
- Used multiple statistical analyses of the primary outcome based on a prespecified statistical analysis plan to ensure robustness of results (i.e. best- and worst-case scenario analyses for patients with missing primary outcome data)
- Randomization occurred 24 hours a day, 7days a week, therefore including all possible patients and not a convenience sample of patients
- Groups were well balanced at baseline, mean MAP at randomization (69.9mmHg in permissive hypotension vs 71.1mmHg in usual care), fluid balance, urine output, or use of pure inotropes
- Clinical management diverged immediately after randomization with a clear difference in management of vasopressors between groups
- The number of patients with one or more occurrences of non-adherence to the protocol was only 153 (11.3%) of patients in the permissive hypotension group with an overall, non-adherence rate of 6% of the total time receiving vasopressors. This shows that using a lower target MAP range is feasible in clinical practice
- This trial also addressed cognitive decline and quality of life among survivors which is an important patient-oriented outcome valued by both patients and families of patients
Limitations:
- This was a non-blinded study, which can result in observer bias. Objective outcomes such as death are at less risk of bias, however, subjective outcomes could be more at risk.
- This was a study of patients ≥65 years of age and therefore its results should not be extrapolated to other patient populations
- The use of permissive hypotension occurred in the ICU, within 6 hours, but it is unclear whether targeting a MAP >60mmHg is what is best practice early in a patient’s resuscitation (i.e. Emergency department).
- Study was powered to detect a 6% absolute risk reduction (approximately 2/3rds of the observed absolute risk reduction in the individual patient data meta-analysis [4]) with an expected 90d mortality in the intervention group of 29%. However, the 90-day mortality was higher than the predicted 29% in the usual care arm (43.8%) and there was more than the 2.5% predicted no consent and withdrawals than anticipated (≈5%)
- 1/3rd of patients who survived three months did not return a completed follow-up health-related quality of life (HrQoL) questionnaire
Discussion:
- Nearly half the patients in this study (46%) had chronic hypertension, 1/3rd of patients came from the emergency department, and the most common vasopressor used was norepinephrine (≈60% of patients)
- Targeting a lower MAP (i.e. >60mmHg vs >65mmHg) may be a more labor-intensive intervention for nurses that does not extrapolate to settings outside the ICU and could potentially expose patients to lower MAPs (i.e. <60mmHg). In logistic terms, in the ICU nurses have a 2 patient to 1 nurse ratio. This is often not the case in the ED and because of this, patients may actually have lower MAPs (i.e. 50s) for longer periods of time
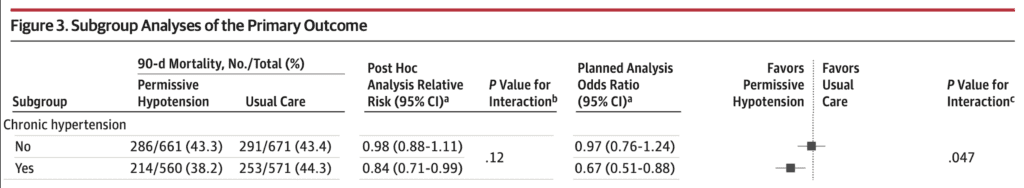
- There was no statistically significant difference in 90d mortality in any of the subgroups with the exception of chronic hypertension:
- Permissive Hypotension: 38.2%
- Usual Care: 44.3%
- Adjusted OR 0.67; 95% CI 0.51 to 0.88; p = 0.47
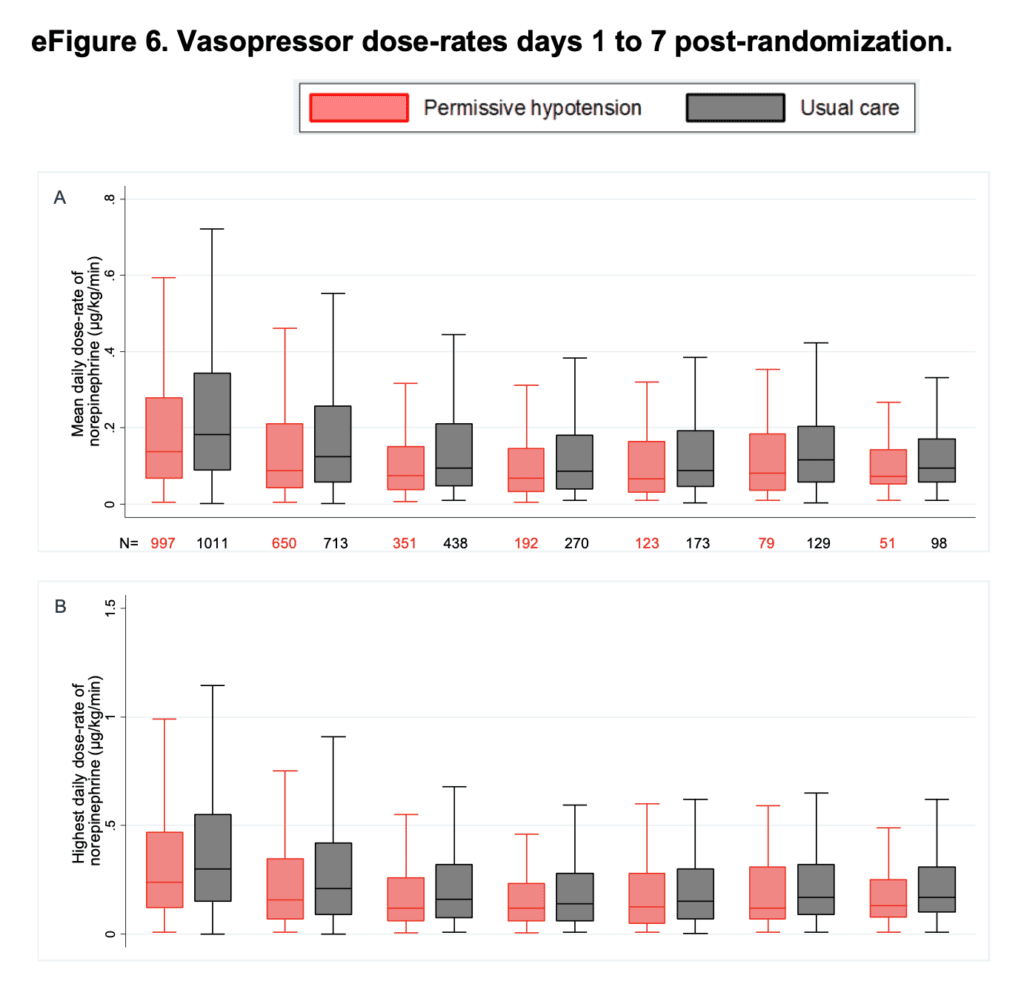
- Summary of the median of the mean and peak (highest) daily dose-rates of norepinephrine equivalents days 1 – 7 post randomization
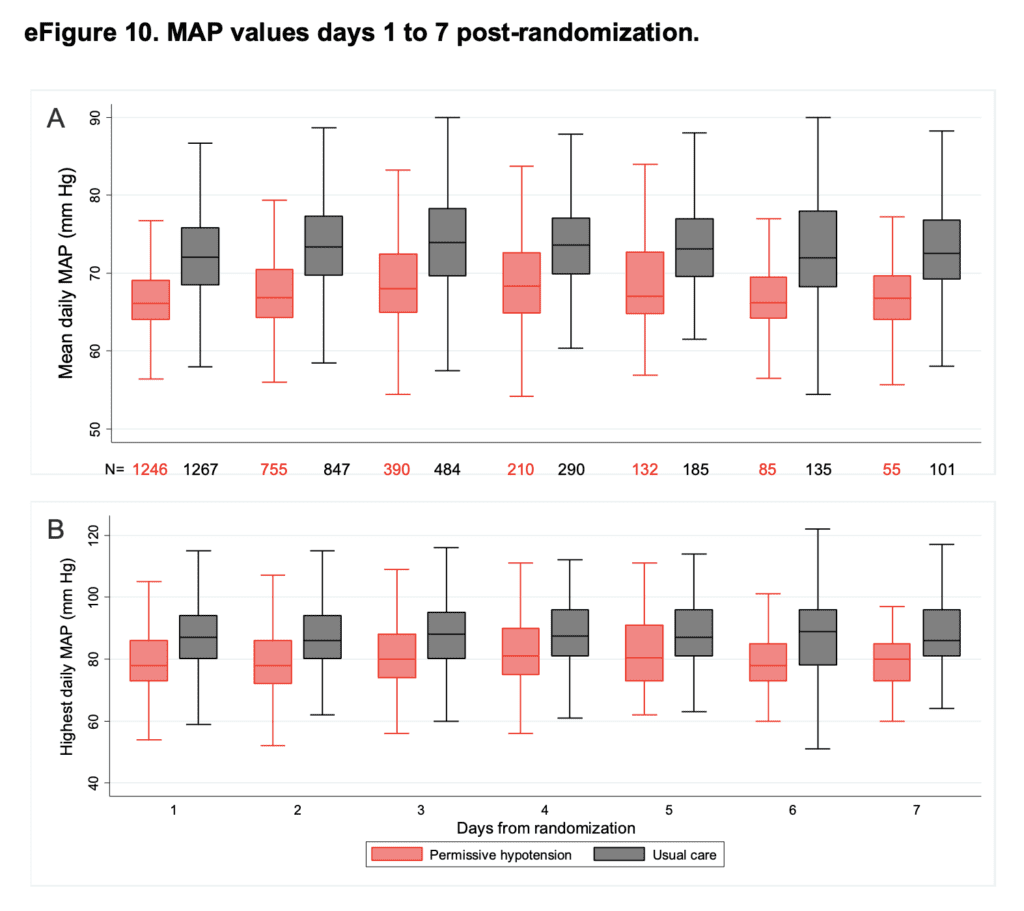
- Median of the mean MAPs and peak (highest) MAPs days 1 – 7 post randomization
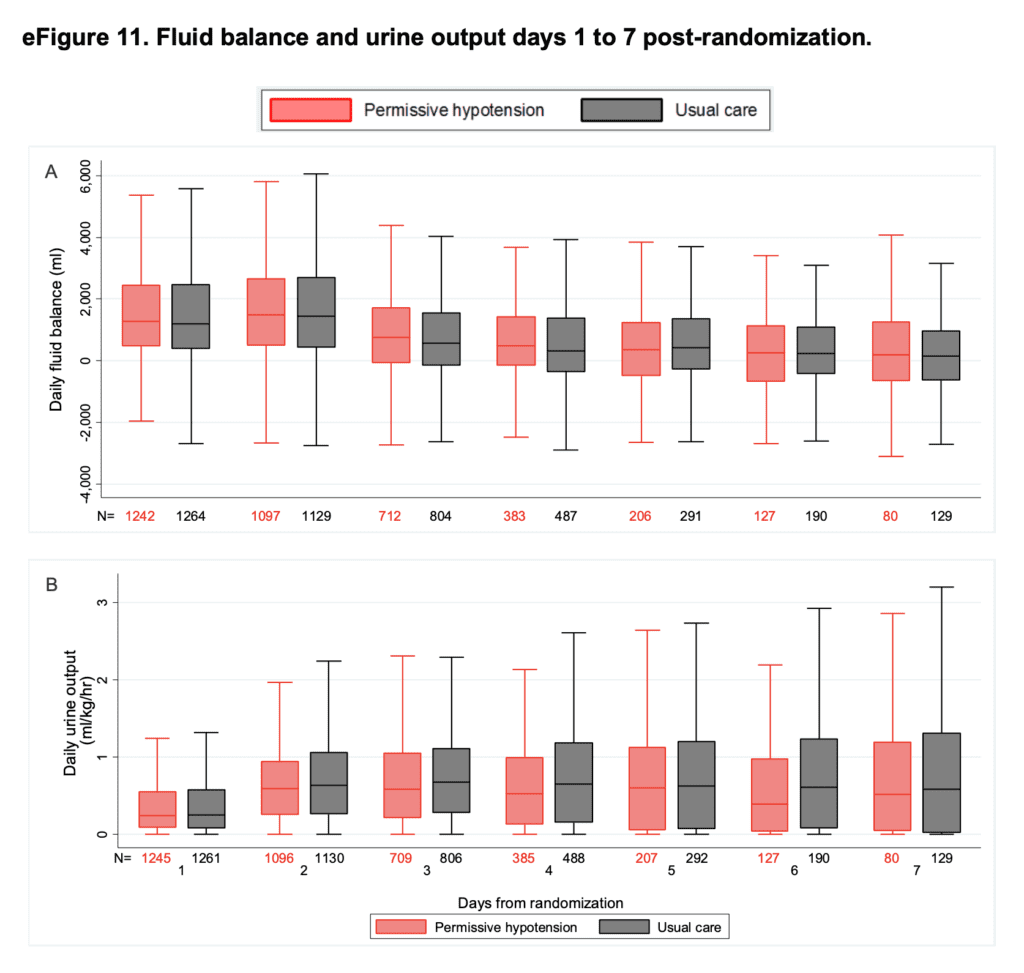
- Median daily fluid balance and daily urine output for days 1 – 7 post randomization
- Currently the Impact of Permissive Hypotension on End-Organ Damage in the Elderly (OVATION-65) Trial, a randomized clinical trial of 200 patients is underway, which may shed more light on these results (Link is HERE)
- Finally, in the accompanying editorial by John C. Marshall [5] his closing paragraph summarizes many of my thoughts on “negative” trials and the 65 Trial…
“Randomized clinical trials yield insight, not instructions. The message of the 65 trial by Lamotagne et al is not that all patients should be treated the same, rather that treatment decisions can be individualized around a somewhat lower mean MAP comfort point. A MAP target of 60 to 65mmHg appears to be more than adequate for most older patients with vasodilatory shock, and contrary to conventional wisdoms, it is possible that a lower target threshold may be more beneficial in older patients with preexisting hypertension. Some “negative” trials can contribute to changes in clinical practice.”
Author Conclusion: “Among patients 65 years or older receiving vasopressors for vasodilatory hypotension, permissive hypotension compared with usual care did not result in a statistically significant reduction in mortality at 90 days. However, the confidence interval around the point estimate for the primary outcome should be considered when interpreting the clinical importance of the study.”
Clinical Take Home Point: There is most likely no one magic number to target as sepsis is a heterogenous disease process requiring individualization of resuscitation with minute to minute monitoring. Furhtermore it is important to realize that MAP or BP alone do not equal perfusion. Although the 65 trial, is a “negative” one, it should assure us that targeting a MAP of >60mmHg in patients ≥65 years IN THE ICU (NOT the ED) is a safe practice in a time of less is more in the management of sepsis (i.e. less fluids, lower hemoglobin cutoffs, and less vasopressors).
References:
- Lamontagne F et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA 2020. PMID: 32049269
- Asfar P et al. High Versus Low Blood-Pressure Target in Patients with Septic Shock. NEJM 2014. PMID: 24635770
- Lamontagne F et al. Canadian Critical Care Trials Group. Higher Versus Lower Blood Pressure Targets for Vasopressor Therapy in Shock: A Multicentre Pilot Randomized Controlled Trial. Intensive Care Med 2016. PMID: 26891677
- Lamontagne F et al. Pooled Analysis of Higher Versus Lower Blood Pressure Targets for Vasopressor Therapy Septic and Vasodilatory Shock. Intensive Care Med 2018. PMID: 29260272
- Marshall JC et al. Choosing the Best Blood Pressure Target for Vasopressor Therapy. JAMA 2020. PMID: 32049266
For More Thoughts on This Topic Checkout:
- EM Lit of Note: Stayin’ Alive Below 65
- The Bottom Line: 65 Trial
- PulmCrit – The 65 Trial – Is 60 the New 65?
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)