Paper: Panos NG et al. Factor Xa Inhibitor-Related Intracranial Hemorrhage: Results From a Multicenter, Observational Cohort Receiving Prothrombin Complex Concentrates. Circulation 2020. PMID: 32264698
Clinical Question: What is the safety and efficacy of prothrombin complex concentrates (PCCs) for factor Xa inhibitor related intracranial hemorrhage (ICH)?
What They Did:
- Multicenter, retrospective, observational cohort study of patients with apixaban or rivaroxaban related ICH who received PCCs
- Pre-planned analysis of patients treated with PCCs for FXa inhibitor related ICH
- 26 participating medical centers (all designated certified stroke centers)
Outcomes:
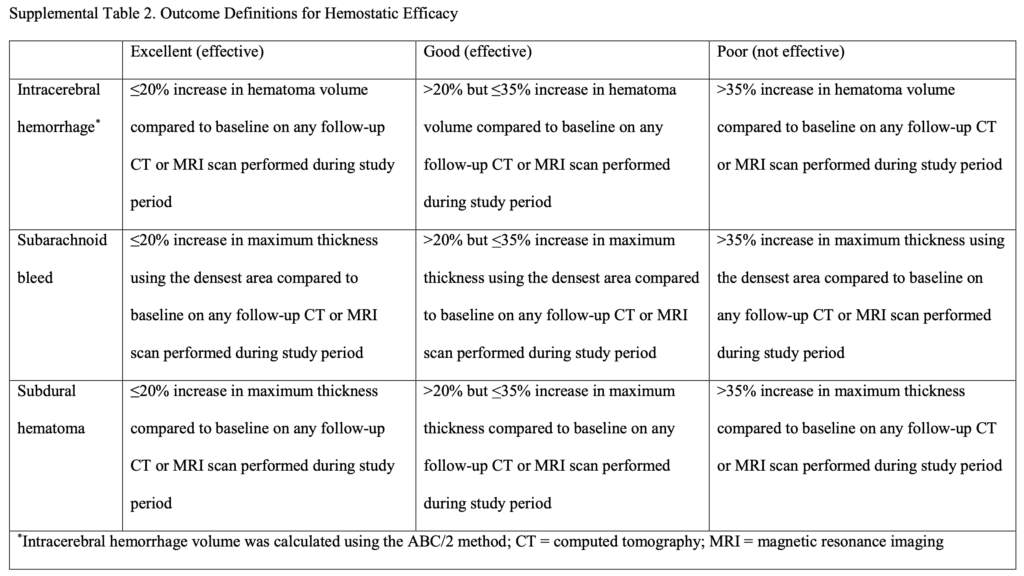
- Primary Efficacy Outcome: Percentage of patients with excellent or good hemostasis
- Excellent hemostasis = 0 to 20% increase in hematoma size
- Good hemostasis = 20.1 to 35.0% increase in hematoma size
- Poor hemostasis = >35.0% increase in hematoma size
- Primary Safety Outcome: Occurrence of thrombotic event during hospitalization
- Thrombotic event = DVT, PE, acute ischemic stroke, MI, or any other documented thrombosis
- Secondary:
- In-hospital mortality
- Length of stay
- Infusion-related reactions
- Thrombotic events in pre-defined periods post-PCC administration (0 -5d, 6 – 14d, and 15 – 30d)
Inclusion:
- ≥18 years of age
- Spontaneous or traumatic ICH (intracerebral, subarachnoid, or subdural)
- Taking apixaban or rivaroxaban in a timeframe before hospital presentation that warranted administration of PCCs
- Received aPCCs or 4-factor PCCs
Exclusion:
- Hemorrhage associated with other anticoagulant agents or liver disease
- Epidural or any intraventricular hemorrhage not included in hemostatic efficacy analysis (lack of clear criteria to assess hemostasis in these hemorrhage types)
- Pregnancy or lactating patients
- Lack of follow-up assessment to assess safety and efficacy due to withdrawal of life-sustaining measures within 24 hours of hospital admission
- Enrolled in any prospective study investigating andexanet alfa
- Prisoners
- Did not have at least 1 follow-up image of the brain within the first 24 hours of PCC administration
Results:
- 707 patients evaluated for inclusion
- 44 patients excluded (most common reason: 35 no repeat brain imaging with 24hr of reversal agent)
- 663 patients included and assessed for safety outcome
- 230 excluded (most common reason: 148 with intracerebral or SAH with intraventricular extension)
- 443 met criteria for hemostatic efficacy evaluation
- Intracerebral hemorrhage = 172
- Subarachnoid hemorrhage = 68
- Subdural hemorrhage = 193
- Important Medication Info:
- Apixaban: 366 patients (55.2%)
- Rivaroxaban: 297 patients (44.8%)
- 4 Factor PCC: 514 patients (77.5%)
- Median initial dose of 43.8 (25.6 to 49.8) units/kg
- Activated PCC: 149 patients (22.5%)
- Median initial dose 26.7 (23.8 to 48.3) units/kg
- Median time to PCC administration from hospital presentation in all 663 patients: 2.6 (1.5 to 4.3) hours
- Second dose of PCC administered to 34 patients (5.1%)
Critical Results:
- Hemostatic Evaluation:
- Excellent or Good Hemostasis: 354 patients (81.8%; 95% CI 77.9 to 85.2)
- Median ICU LOS: 2.0d
- Median Hospital LOS: 6.0d
- Safety Evaluation:
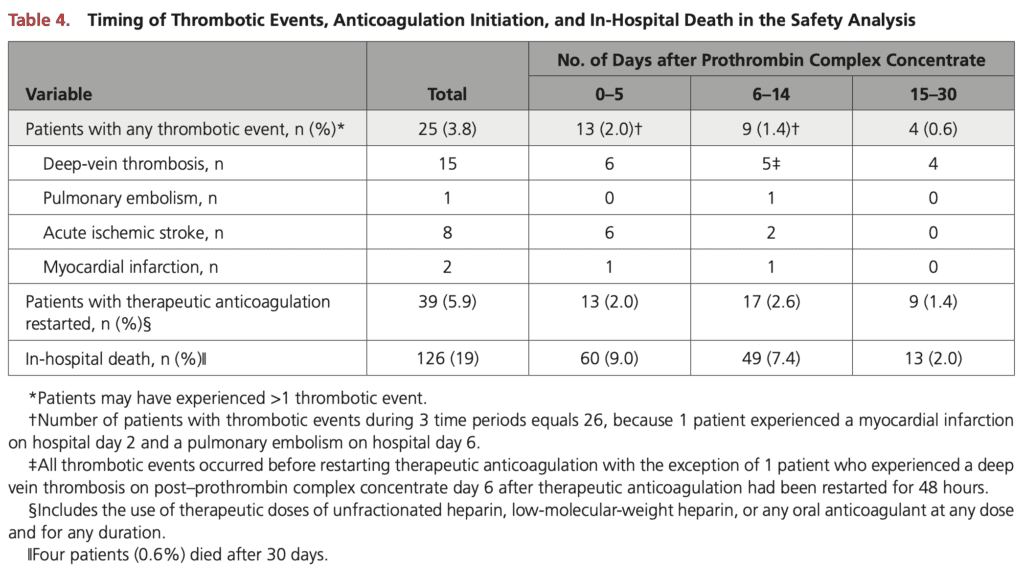
- Thrombotic Events: 26 patients (3.8%)
- 22 occurred in the first 14 days after PCC administration
- 8 patients had an acute ischemic stroke found on repeat brain imaging (all patients had a history of atrial fibrillation)
- aPCC: 8 out of 149 patients had 9 thrombotic events (6 DVT, 1 PE, 2 MI)
- 4 Factor PCC: 17 out of 514 patients had 18 thrombotic events (9 DVT, 8 ischemic stroke)
- Infusion-Related Reaction: 1 patient
- In-Hospital Mortality: 19.0%
- Thrombotic Events: 26 patients (3.8%)
Strengths:
- Largest multicenter, observational study to date to evaluate hemostatic efficacy and safety of PCCs in patients with apixaban- or rivaroxaban-related ICH
- All participating medical centers were AHA/ASA stroke centers which helps reduce variations in standard care amongst sites
Limitations:
- Observational cohort study which allowed for variability in treatment practices between sites (i.e. dose and type of PCC used)
- Unclear how well established Sarode criteria is. No inter-rater reliability of the score which is important as it doesn’t seem anyone was blinded to the fact that PCC was given
- The primary outcome was not a patient centered one
- Unclear if patients actually took their Xa inhibitor as no assay of Xa activity was done
- No comparison group. We may have seen similar results in a placebo group
- Unable to evaluate hemostatic efficacy on 230 patients in the safety analysis due to intraventricular hemorrhage, intracerebral or subarachnoid hemorrhage with intraventricular extension, and undergoing surgical procedures prior to follow-up imaging. The exclusion of these patients could potentially affect the hemostatic efficacy observed
- Unable to record exact timing of last apixaban or rivaroxaban dose due to lack of uniform documentation across all participating sites
- No assessment of correlation between hemostatic efficacy and anti-Xa activity levels
- No evaluation of agent used or timing of VTE prophylaxis initiation
- Hawthorne Effect: Everyone knew they were trying to study PCC for reversal with no comparator so everyone could have erred toward it working and scoring patients favorably
Discussion:
- Study took place over 51 months. Recruitment of 663 patients during this time period would mean about 13 patients per month with Xa inhibitor ICH receiving PCC
- The primary indication for anticoagulation in this study was atrial fibrillation (≈80% of patients)
- This same group of authors is also evaluating the use of andexanet alfa in patients on Xa inhibitors, but due to the limited number of participating sites using andexanet alfa, data collection is still ongoing
- For patients with multiple intracranial hemorrhages, the largest bleeding site was used as the primary hemorrhage site
- Although hemostatic efficacy was to be assessed in the first 24 hours after PCC treatment, the median time to first follow-up imaging was 6.1hours. This may overexaggerate hemostatic effects compared to imaging being done at 24 hours. 120 patients had a second follow-up image during the study period at a median time of 14.2hrs
- Thromboembolic events can occur after PCC administration for multiple reasons other than the administration of the medication itself such as stopping anticoagulation abruptly in patients with medical conditions that predispose them to the development of thromboembolism
- Thromboembolic events can occur out to about 14 days after treatment with PCCs (Factor II: 60-hour half-life = 5 half-lives = about 14 days)
- Most previous evidence on in-hospital mortality rates are about 20 – 22%. This current trial has an in-hospital mortality rate (19.0%) that is similar to previous studies
Author Conclusion: “Administration of PCCs after apixaban- and rivaroxaban-related ICH provided a high rate of excellent or good hemostasis (81.8%) coupled with a 3.8% thrombosis rate. Randomized, controlled trials evaluating the clinical efficacy of PCCs in patients with factor Xa inhibitor-related ICH are needed.”
Clinical Take Home Point: There is a true lack of prospective randomized clinical trials with respect to apixaban- and rivaroxaban-related ICH. This observational trial unfortunately doesn’t give us any real clinical guidance as there is no comparator arm and we simply don’t know what patient-oriented outcomes would be. Additionally, although the study tells us there is a low rate of VTE, we have no idea how robustly the authors screened for VTE (i.e. if you don’t look, you may not find VTE). Finally, physicians should be cautious of thromboembolic events out to 14 days after using PCCs.
References:
- Panos NG et al. Factor Xa Inhibitor-Related Intracranial Hemorrhage: Results From a Multicenter, Observational Cohort Receiving Prothrombin Complex Concentrates. Circulation 2020. PMID: 32264698
- Frontera JA et al. Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: A Statement for Healthcare Professionals From the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care 2016. PMID: 26714677
For More Thoughts on This Topic Checkout:
- REBEL EM: ANNEXA-4: Andexanet Alfa and Factor Xa Inhibitors
- REBEL EM: Rebellion in EM 2018 – DOAC Reversal by Scott Wieters, MD
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)