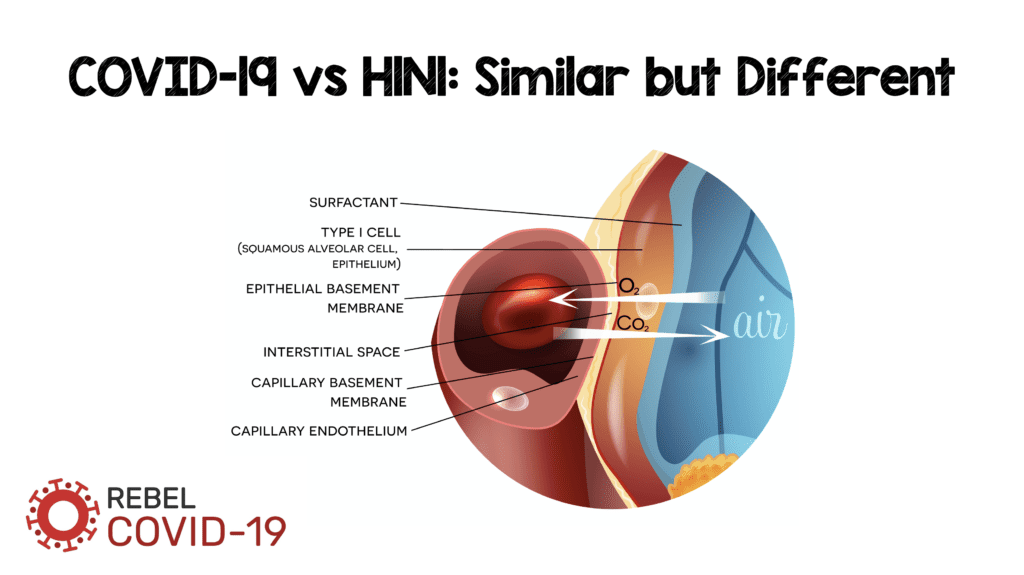
Understanding of the mechanisms of COVID-19 is badly needed if we are to find treatments that may be beneficial. The leading cause of mortality in patients with COVID-19 is hypoxemic respiratory failure most frequently resulting in ARDS. However, the mechanisms that bring patients from infection to ARDS are unknown: is it diffuse alveolar damage (DAD), endothelial damage, or some combination of both? Although it may seem ridiculous to consider these two entities as separate, as the alveolar-capillary interface is submicrons in size, we want to know if one of these two entities is driving the injury more than the other? There have been some interesting pathological reports that have been published looking at the histopathology of COVID-19, and many more discussions about the similarities to other viral pneumonias (i.e. H1N1). A recent publication in NEJM compares the pathology of COVID-19 vs H1N1.
Below is an image from Teuwen LA et al [2] depicting a normal and abnormal interface between the alveolus and endothelial cells
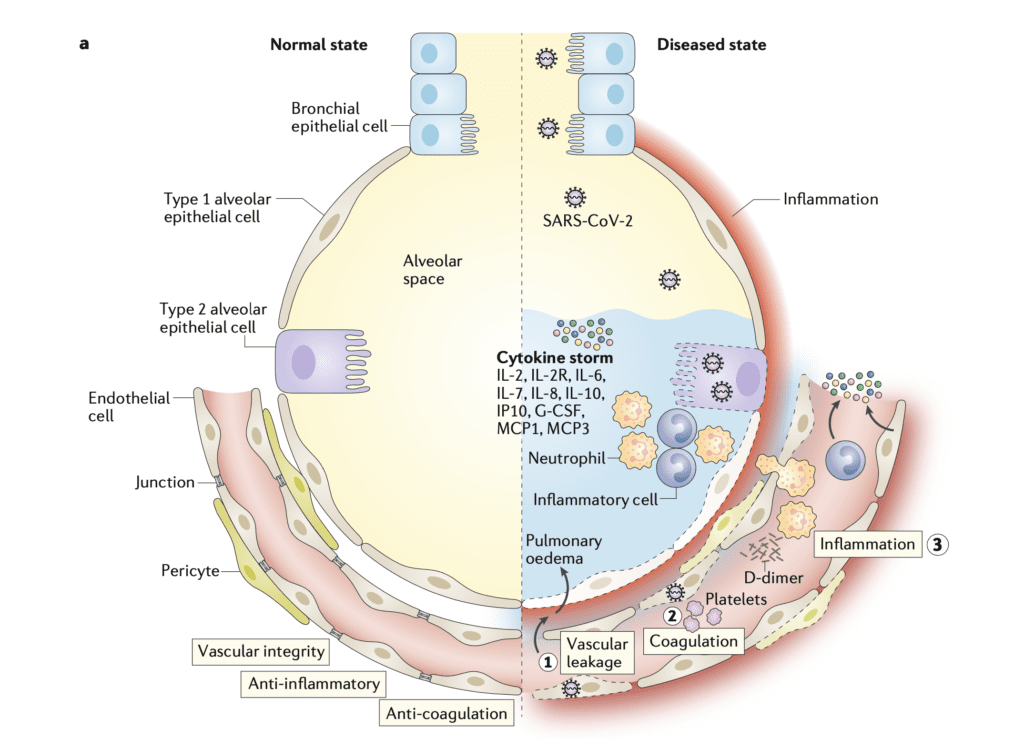
The image above sets the stage to discuss several potential mechanisms of injury. In the rest of the post we will review a pathological study comparing the morphologic characteristics of COVID-19, H1N1, and case matched lungs. Although the Teuwen LA et al model is a proposed model of vascular leakage, coagulation, inflammation, and angiogenesis, the Ackermann M et al publication [1] shows us how H1N1 and COVID-19 are similar, yet different.
Article: Ackermann M et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. NEJM 2020. [Epub Ahead of Print]
What They Did:
- Autopsy study of:
- Patients who died of COVID-19: 7 lungs
- Patients who died from ARDS secondary to A(H1N1): 7 lungs (Archived lung tissue from the 2009 pandemic)
- Age-matched, uninfected control lungs: 10 lungs
- All lungs were studied with various manners of analysis
Histology of Acute Respiratory Distress Syndrome (ARDS)
- Diffuse alveolar damage (DAD) with edema, hemorrhage, and intraalveolar fibrin deposition
- DAD is a nonspecific finding and can occur from many possible causes, both infectious and non-infectious
- The SARS-CoV and SARS-CoV-2 versions of DAD also include reports of fibrin thrombi
Mean Weight of Lungs:
- H1N1 > COVID-19 > Matched Controls
- The increased weight of the lungs in COVID-19 and H1N1 is most likely from vascular leakage and pulmonary edema. Some of the possible mechanisms for this are described by Teuwen LA et al [2]:
- Virus can directly affect endothelial cells resulting in endothelitis, lysis and death
- SARS-CoV-2 binds to ACE2 receptors which impair its activity by downregulation or shedding. Reduced ACE2 activity indirectly activates kallikrein-bradykinin pathways which increase vascular permeability
- Activated neutrophils produce histotoxic mediators (i.e. reactive oxygen species)
- Immune cells, inflammatory cytokines, and vasoactive molecules lead to enhanced endothelial contractility and loosen the inter-endothelial junctions leading to gaps
- IL-1B and TNF activate glucuronidases that degrade the glycocalyx and upregulate hyaluronic acid synthase 2, leading to increased hyaluronic acid deposition in the extracellular matrix and promote fluid retention
- The net effect of all of these mechanisms is increased vascular permeability and vascular leakage
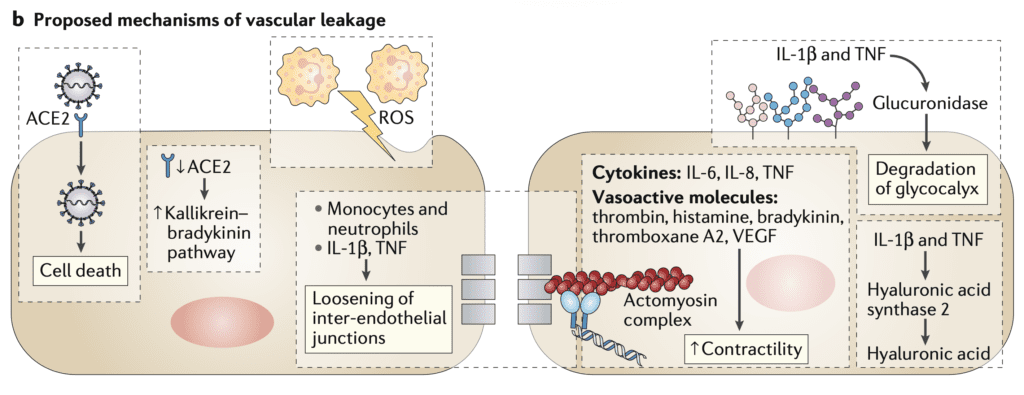
Proposed Mechanism of Vascular Leakage (Image from [2]
Angiocentric Inflammation
- Both COVID-19 and H1N1 had DAD with necrosis of alveolar lining cells and intraalveolar fibrin deposition
- COVID-19: The changes were more focal in most cases and only had mild interstitial edema
- H1N1: The changes were more diffuse with massive interstitial edema and extensive fibrin deposition in all cases
- This helps explain the higher weight of the lungs with H1N1 compared to COVID-19
- Mean Angiotensin-Converting Enzyme 2 (ACE2) Expression (ACE2 positive cells per field of view):
- There are significantly more ACE2 positive cells in the capillary endothelial cells than the alveolar epithelial cells in both COVID-19 and H1N1
- Additionally, there were ACE2-positive lymphocytes seen in the perivascular tissue as well as the alveoli in both COVID-19 and H1N1
- Increased vascular inflammation is present in both COVID-19 and H1N1, however appears to be more predominant in capillary endothelial cells (This explains the increase in MI, stroke, PE etc…seen in both diseases)
Thrombosis and Microangiopathy
- Fibrin microthrombi could be seen in the alveolar capillaries in all the lungs from both COVID-19 and H1N1
- Alveolar capillary microthrombi were 9x more prevalent in patients with COVID-19 vs H1N1
- The authors also performed three-dimensional microCT of the pulmonary specimens which confirmed the histologic findings that both COVID-19 and H1N1 showed occlusions of precapillary and postcapillary vessels
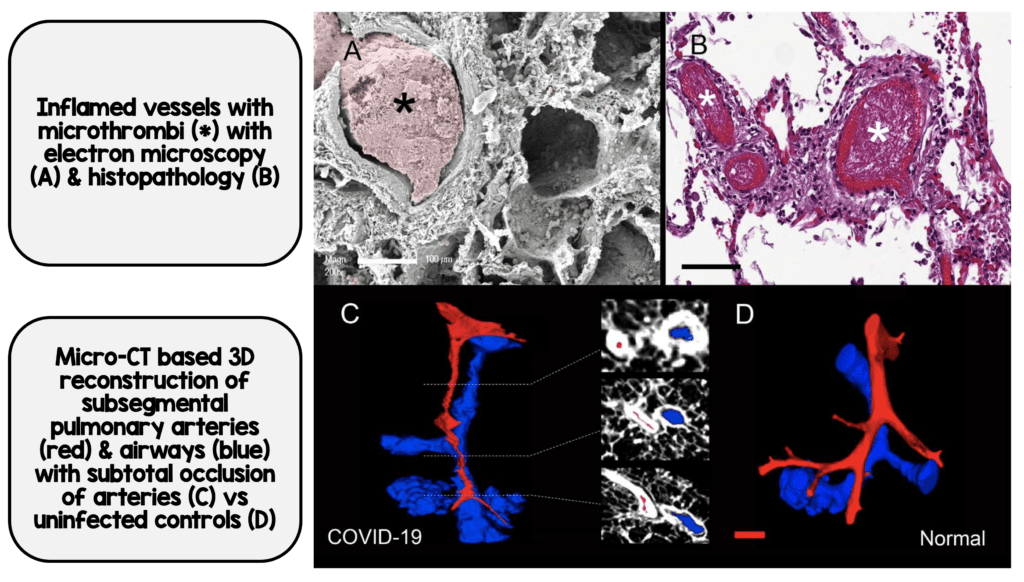
COVID-19 Associated Thrombosis (Image from [1])
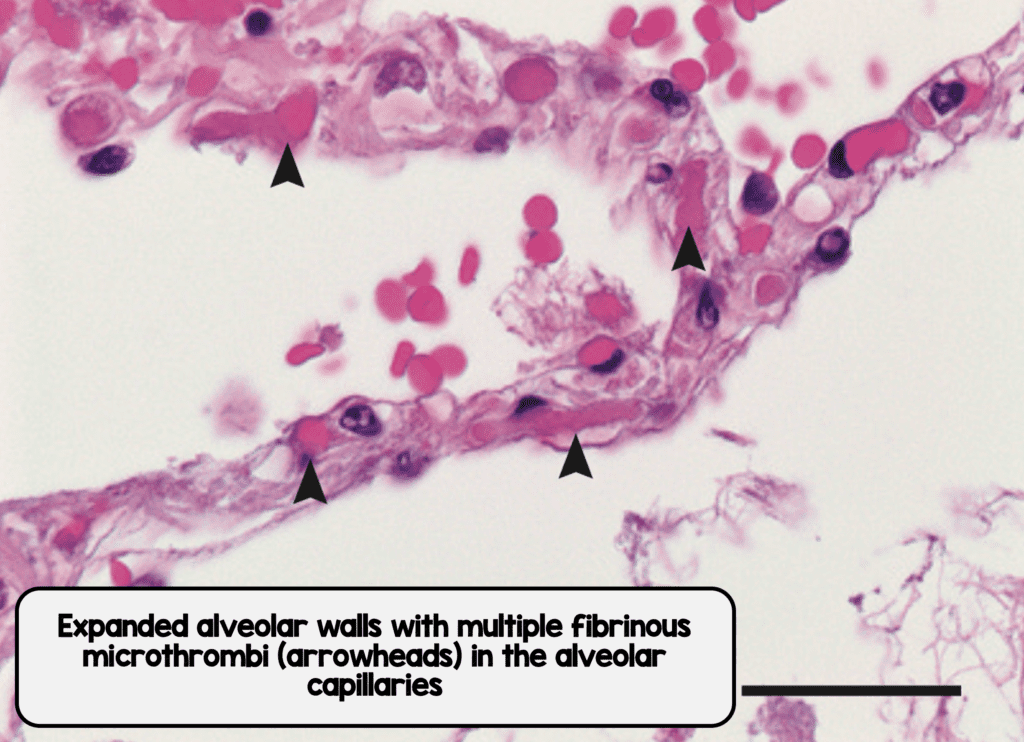
Microthrombi in Interalveolar Septa from COVID-19 (Image from [1]
- Teuwen LA et al [2] propose a set of mechanisms as to why we see so much thrombosis in COVID-19
- Disruption in vascular integrity and endothelial cell death leads to exposure of the thrombogenic basement membrane. This then activates the clotting cascade
- IL-1B and TNF increase the expression of P-selecting, von Willebrand factor and fibrinogen (These are what platelets bind to)
- Endothelial cells release trophic cytokines which can augment platelet production. Platelets release VEGF, which triggers endothelial cells to upregulate tissue factor (prime activator of the coagulation cascade)

Proposed Mechanisms of Coagulation Initiation (Image from [2])
- Both COVID-19 and H1N1 lungs had microthrombi present, in the pre-capillary, alveolar capillary, and post capillary venules of varying degrees, but with a significantly higher prevalence seen in COVID-19 in the alveolar capillaries (This could explain why standard prophylactic anticoagulation may not work in some patients)
Angiogenesis
- COVID-19: Distorted vascularity with structurally deformed capillaries. Elongated capillaries with sudden changes in caliber and intussusceptive pillars within the capillaries. Ultrastructural damage to endothelium with both intra- and extra-cellular SARS-CoV-2
- Mean density of intussusceptive and sprouting angiogenic features were higher in COVID-19 vs H1N1
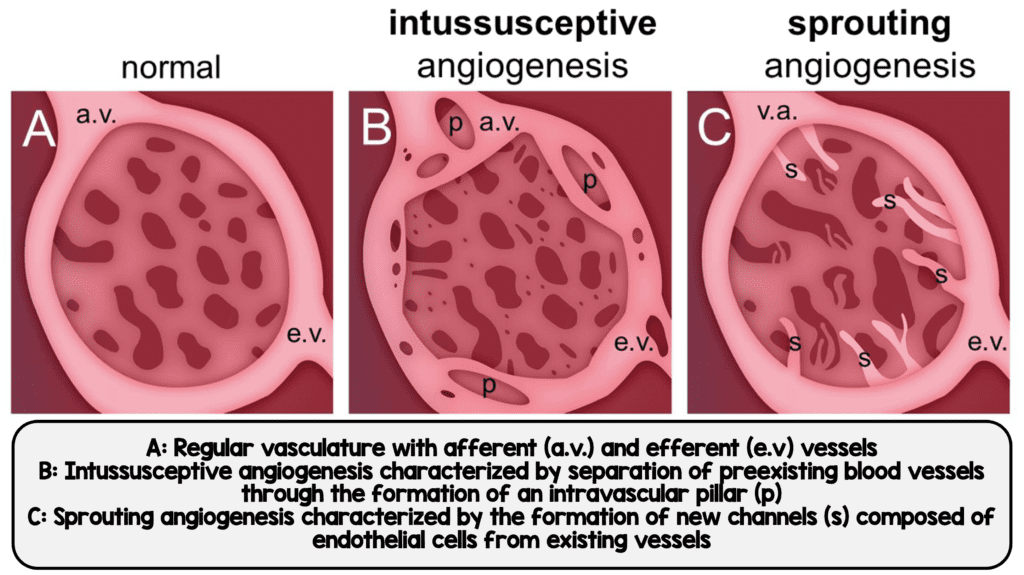
Intussusceptive and Sprouting Angiogenesis seen in COVID-19 (Image from [1])

Electron Micrographs Showing Microvascular Alterations in COVID-19 Lungs (Image from [1])
- Teuwen LA et al [2] propose that due to increased microthrombosis seen with COVID-19, lung tissue ischemia begins to develop which triggers angiogenesis and endothelial cell hyperplasia
- Pulmonary Angiogenic Counts vs Length of Hospital Stay
- Intussusceptive angiogenesis and sprouting angiogenesis were significantly more profound compared to H1N1 and matched controls (See image below)

Density of Intussusceptive and Sprouting Angiographic Features of COVID-19, H1N1, and Matched Controls (Panels A & B) and vs Hospitalization Time (Panels C & D) (Image from [1])
- Increased intussusceptive and sprouting angiographic features are seen in both COVID-19 and H1N1, however the density of these features is significantly more in COVID-19 (This is typically due to hypoxia and growth factors)
Important Limitations:
- A major limitation of this study is that none of the COVID-19 patients were mechanically ventilated whereas 5 out of 7 patients in the H1N1 group were.
- The differences in angiogenesis could also be due to the different time courses of COVID-19 and influenza which cannot be controlled for in this type of trial
Summary of Findings:
- The lungs of patients with COVID-19 and H1N1 have some overlapping similarities and some major differences:
- Both diseases have DAD and infiltrating perivascular lymphocytes and a greater number of ACE-2 positive cells in the lungs (Predominantly in the capillary endothelial cells)
- However, COVID-19 differs from H1N1 in several distinct ways:
- Severe endothelial injury with disrupted endothelial cell membranes
- Widespread vascular thrombosis with microangiopathy and occlusion of alveolar capillaries (9x more prevalent compared to H1N1)
- More new vessel growth (Intussusceptive and sprouting angiogenesis)
Additional Thoughts:
- We know that patients who are older, obese, have hypertension and diabetes mellitus are at higher risk of more severe disease. Why might this be? What do they all have in common?…They are all characterized by pre-existing vascular dysfunction and altered endothelial cell metabolism.
Bottom Line:
- Both groups of patients have signs of tissue hypoxia, however, there appears to be a greater degree of endothelialitis and thrombosis in the lungs of patients with COVID-19 vs H1N1 (This could be why prophylactic anticoagulation may not be enough in COVID-19, but still remains to be proven)
- There is overlap between the two diseases, but it seems COVID-19 appears to be more of an endothelial rather than an alveolar disease process as the primary driver (Future studies and discussion should look at treatments focused on this aspect of disease)
References:
- Ackermann M et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. NEJM 2020. [Epub Ahead of Print]
- Teuwen LA et al. COVID-19: The Vasculature Unleashed. Nature Reviews Immunology 2020. [Epub Ahead of Print]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)





