What Trial are we Reviewing:
Baharoglu MI et al. Platelet Transfusion Versus Standard Care After Acute Stroke due to Spontaneous Cerebral Haemorrhage Associated with Antiplatelt Therapy (PATCH): A Randomised, Open-Label, Phase 3 Trial. Lancet 2016; 1 – 9 [epub ahead of print]
What They Did:
- Multicenter, Open-Label, Masked-Endpoint, Randomized Trial
- 60 Hospitals in the Netherlands, UK, and France
- Enrolled patients ≥18years of age with non-traumatic supratentorial ICH confirmed by brain imaging and Glasgow Coma Scale (GCS) of at least 8 – 15 in which platelet transfusion could be initiated within 6hrs of symptom onset and within 90 min of brain imaging who had been on antiplatelet therapy
- Randomized to standard care or standard care with platelet transfusion within 90 minutes of allocation
Inclusion Criteria:
- Antiplatelet Therapy for at least 7 days preceding ICH =
- Cyclooxygenase (COX) inhibitors (Aspirin, or Carbasalate Calcium)
- Adenosine Diphosphate (ADP) Receptor Inhibitor (Clopidogrel)
- Adenosine Reuptake Inhibitor (Dipyridamole)
- Pre-ICH modified Rankin Scale (mRS) Score of 0 – 1
Exclusion Criteria:
- Subdural or Epidural Hemorrhage on Brain Imaging
- Underlying Aneurysm or Arteriovenous Malformation
- Planned Surgical Evacuation of ICH within 24hr of Admission
- Known Use of Vitamin K Antagonist (Unless INR ≤1 – 3)
- History of Coagulopathy
- Known thrombocytopenia (<100 cells x 109/L)
- Infratentorial or Large Intraventricular Hematomas
Outcomes:
- Primary: Shift towards death or difference in functional outcome at 3 months after randomization rated on a modified Rankin Scale (mRS)
- Secondary:
- Survival (mRS 1 – 5) at 3 months
- Poor outcome defined as mRS 4 – 6
- Median Absolute Intracerebral hemorrhage growth in mL after 24h on brain imaging
- Safety Outcomes:
- Complications of platelet transfusion (transfusion reactions, and/or thrombotic complications)
- Serious Adverse Events – Complications of Intracerebral hemorrhage (Enlargement, intraventricular extension, hydrocephalus, edema, or brain herniation), epileptic seizures,, infection (urinary tract or pneumonia)
Results:
- 190 patients enrolled
- 97 Platelet Transfusion
- 93 Standard Care
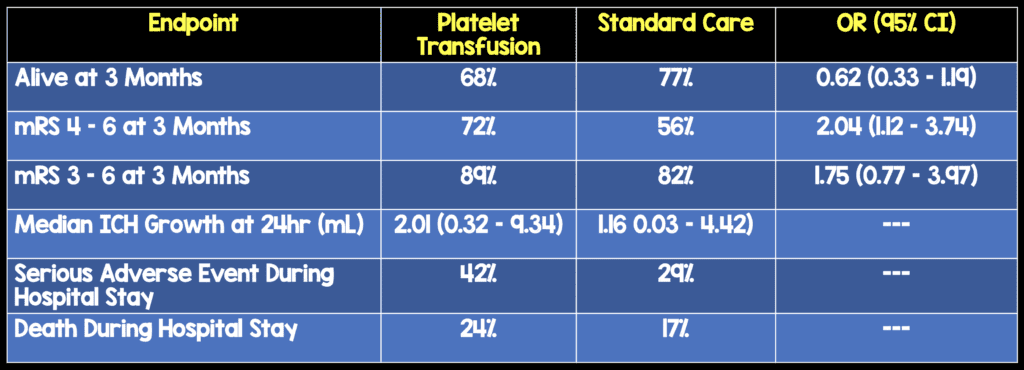
- Odds of Death or Dependence at 3 Months
- Higher in the Platelet Transfusion Group (Adjusted OR 2.05; 95% CI 1.18 – 3.56; p = 0.0114)
Strengths:
- PATCH is the first randomized trial to investigate the effects of platelet transfusion on acute intracerebral hemorrhage after the use of antiplatelet therapy
- Patients were randomized in a 1:1 ratio via a secure, web-based, computerized randomization system that concealed allocation
- Allocation was concealed to outcome assessors and investigators analyzing data
- All diagnostic and 24h brain imaging studies were anonymized and analyzed at a location other than the local site of evaluation
- No patients were lost to follow-up at 3 months and all were included in the final analyses
- Baseline characteristics balanced between groups
Limitations:
- Patients and investigators giving interventions were not masked to treatment allocation
- Four protocol violations occurred in the platelet transfusion group ( 2 patients did not get the correct number of platelets and 2 received platelets out of the prespecified time window)
- Although this study did reach its target sample size, the trial size was smaller than some other acute stroke trials which could cause some chance imbalances in baseline prognostic variables
- Most patients had taken aspirin and not ADP inhibitors which could decrease the generalizability to patients taking these medications
Discussion:
- Based on this trial it appears that platelet transfusion increase the risk of death or dependence in patients with an acute intracerebral hemorrhage while taking antiplatelet therapy
- The findings of this study contrast the hypothesized mechanism of action of platelet transfusion. The mechanism for this is not exactly clear from this study, but some potential mechanisms include:
- Some patients may have had hemorrhagic transformation of infarction rather than intracerebral hemorrhage. Collateral perfusion around the intracerebral hemorrhage could have been impaired resulting in cerebral ischemia. Therefore platelet transfusion could then increase the risk of thrombosis
- Platelets have proinflammatory effects and transfusions might enhance vascular permeability associated with inflammation
- Platelet transfusion might not be beneficial because the absolute increase in intracerebral hemorrhage growth associated with antiplatelet therapy is not large enough to be meaningfully modified by platelet transfusion or platelet transfusion is insufficient to reverse the effects of antiplatelet therapy
Author Conclusion: “Platelet transfusion seems inferior to standard care for people taking antiplatelet therapy before intracerebral hemorrhage. Platelet transfusion cannot be recommended for this indication in clinical practice.”
Clinical Take Home Point: To date this is the best evidence available, and based on this trial, platelet transfusion should be held in patients with acute non-traumatic intracerebral hemorrhage in patients taking antiplatelet therapy as patient outcomes seemed to be worsened.
References:
- Baharoglu MI et al. Platelet Transfusion Versus Standard Care After Acute Stroke due to Spontaneous Cerebral Haemorrhage Associated with Antiplatelt Therapy (PATCH): A Randomised, Open-Label, Phase 3 Trial. Lancet 2016; 1 – 9 [epub ahead of print]
For More Thoughts on This Topic Checkout:
- Ryan Radecki on EMLit of Note: Put the Platelets Away in ICH
- Simon Carley at St. Emlyn’s: JC – Platelets for Intracranial Haemorrhage
- David Slessor at The Bottom Line: PATCH
- Ken Milne at The SGEM: SGEM #182 – Platelet Transfusions for Intracerebral Hemorrhage (PATCH) – Don’t Do It
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



