
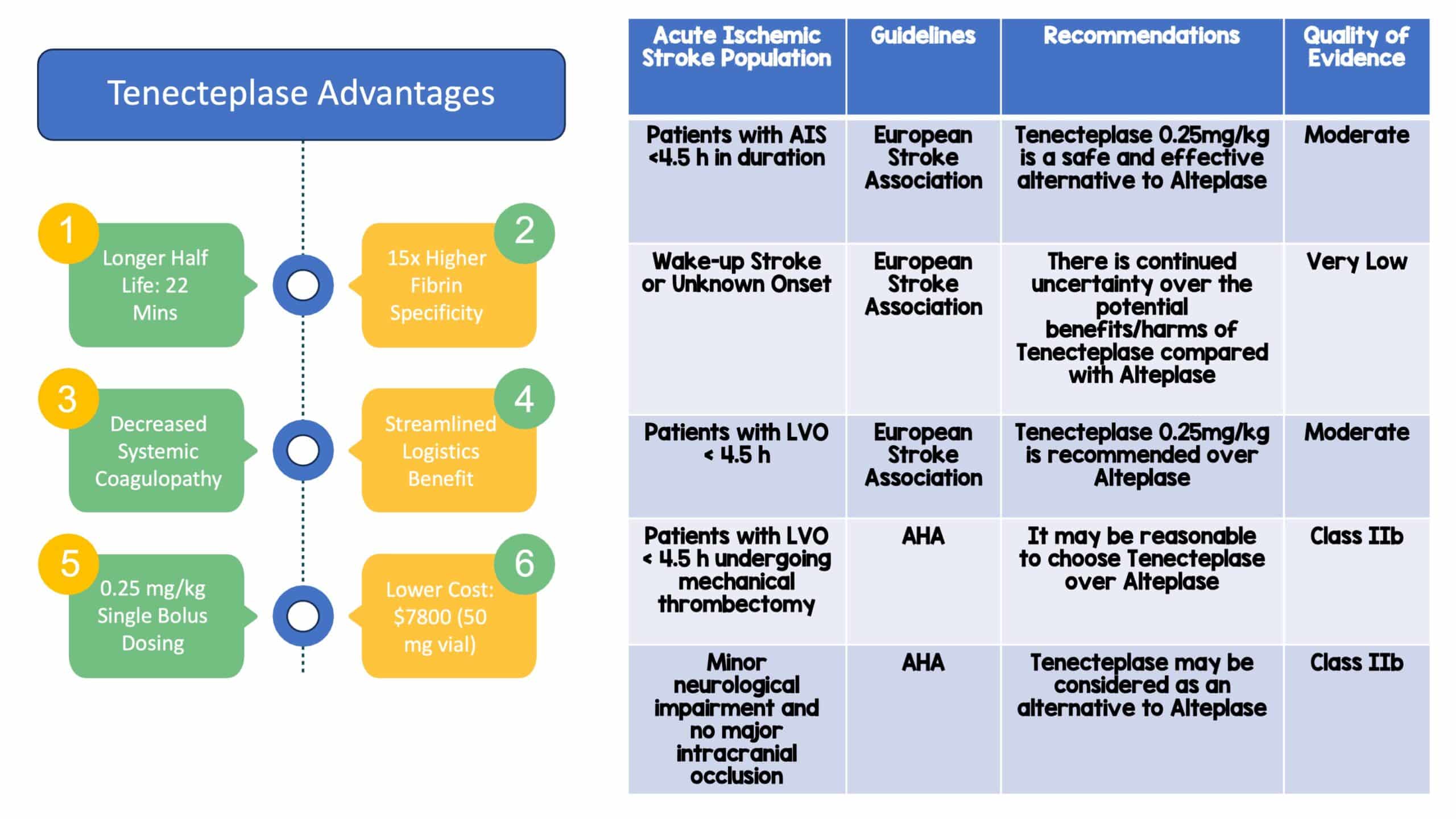
Paper: Memon BK et. Intravenous tenecteplase compared with alteplase for acute ischemic stroke in Canada (AcT): a pragmatic, multicenter, open-label, registry-linked, randomized, controlled, non-inferiority trial. Lancet. 2022. PMID: 35779553
Clinical Question: In adult patients meeting criteria for thrombolysis, is intravenous tenecteplase at a dose of 0.25 mg/kg non-inferior to alteplase in the treatment of acute ischemic stroke?
What they Did:
- Investigator-initiated, Multicenter, Parallel-ground, Open-label, Registry-linked, Randomized, Controlled, Non-Inferiority trial
- 22 Canadian Primary and Comprehensive Stroke Centers from December 2019 to January 2022
- Eligible ischemic stroke patients randomized 1:1 to receive intravenous tenecteplase (0.25 mg/kg, max 25mg) vs. intravenous alteplase (0.09 mg/kg followed by 0.81 mg/kg, max 90 mg) using a balanced allocation algorithm
Outcomes:
- Primary: Proportion of patients with a modified Rankin Scale (mRS) of 0-1 (excellent functional outcome) at 90-120 days
- Non-inferiority margin was set at -5%
- Secondary:
- Proportion of patients with mRS scores of 0-2 at 90-120 days
- 90-120 day EQ-VAS and EQ-5D-5L
- Door to needle time
- Proportion of patients given endovascular therapy
- Recanalization status at first angiographic acquisition in patients undergoing endovascular therapy
- Baseline CT to arterial puncture time in patients undergoing endovascular therapy
- Cognition assessment
- Hospital length of stay
- Discharge destination
- Secondary Safety Analysis:
- Within 24 Hours: Symptomatic ICH, orolingual angioedema, and extracranial bleeding requiring blood transfusion
- 90-day all-cause mortality
Inclusion:
- 18 years or older
- Diagnosis of acute ischemic stroke causing disabling neurological deficit
- Presentation within 4.5 hours of symptom onset
- Eligible for thrombolysis
- Patients eligible for thrombolysis plus endovascular thrombectomy were also included
- Pregnant patients could be enrolled after consultation with an expert stroke physician
Exclusion:
- Patients with standard contraindications to thrombolysis
Results:
- 1577 patients included in intention-to-treat analysis
- Tenecteplase (n=806) vs Alteplase (n=771)
- 10 patients lost to follow up
- Well balanced patient characteristics
- Median age 74 years
- Male patients (52.1%) vs Female patients (47.9%)
- Baseline NIHSS Score
- Tenecteplase: 9
- Alteplase: 10
- Stroke symptom onset to hospital arrival
- Tenecteplase: 82 mins
- Alteplase: 83 mins
- Stroke symptom onset to randomization
- Tenecteplase: 121 mins
- Alteplase: 123 mins
- Door to Needle Time
- Tenecteplase: 36 mins
- Alteplase: 37 mins
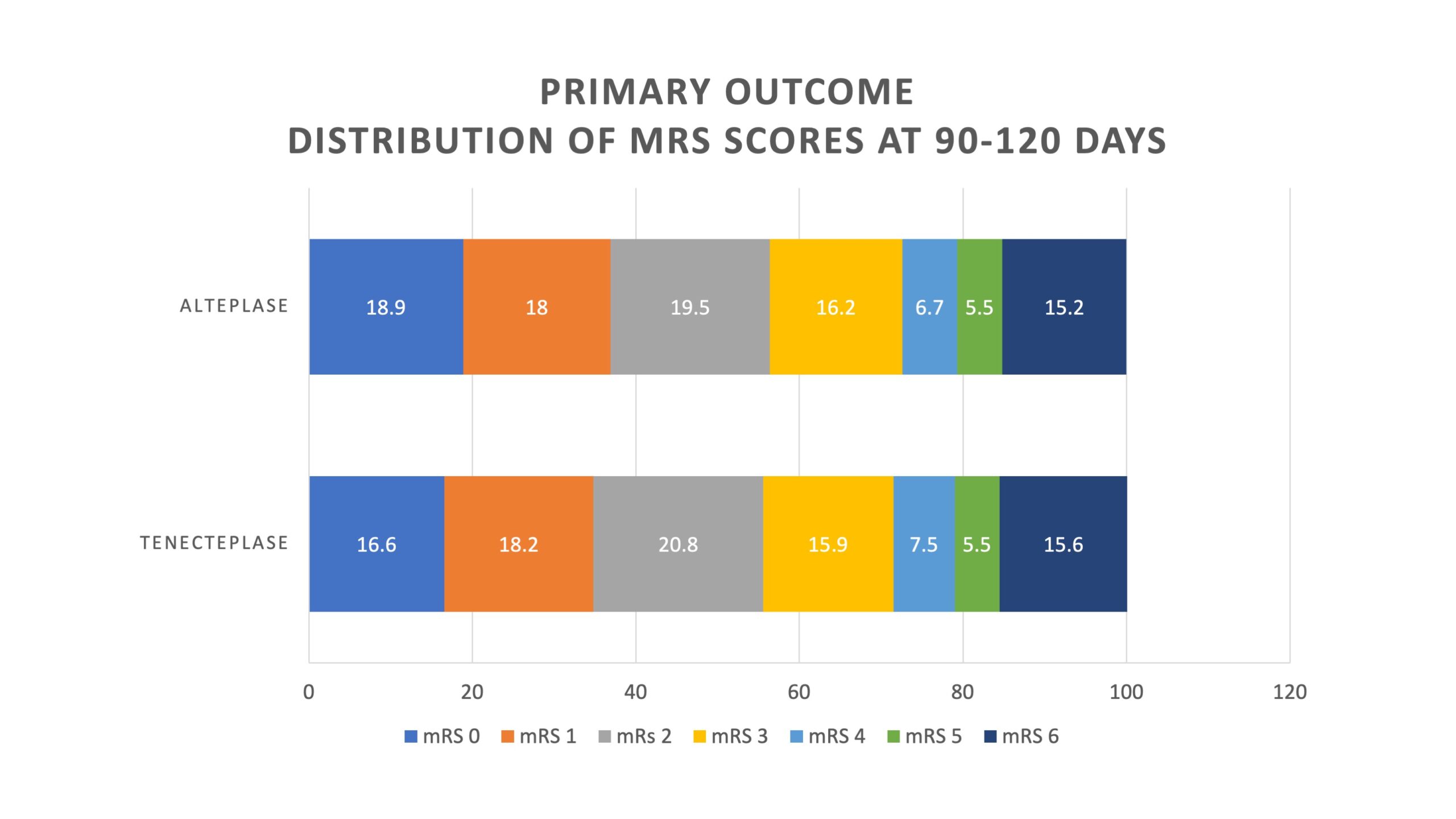
- Primary Outcome: mRS score 0-1 at 90-120 days
- Tenecteplase 36.9% vs Alteplase 34.8%
- Unadjusted risk difference 2.1% [95% CI -2.6 to 6.9]
- The lower bound 95% CI (-2.6%) was greater than -5% (non-inferiority threshold) and thus criteria for non-inferiority were met
- Secondary Outcomes:
- No significant differences noted in any secondary outcome
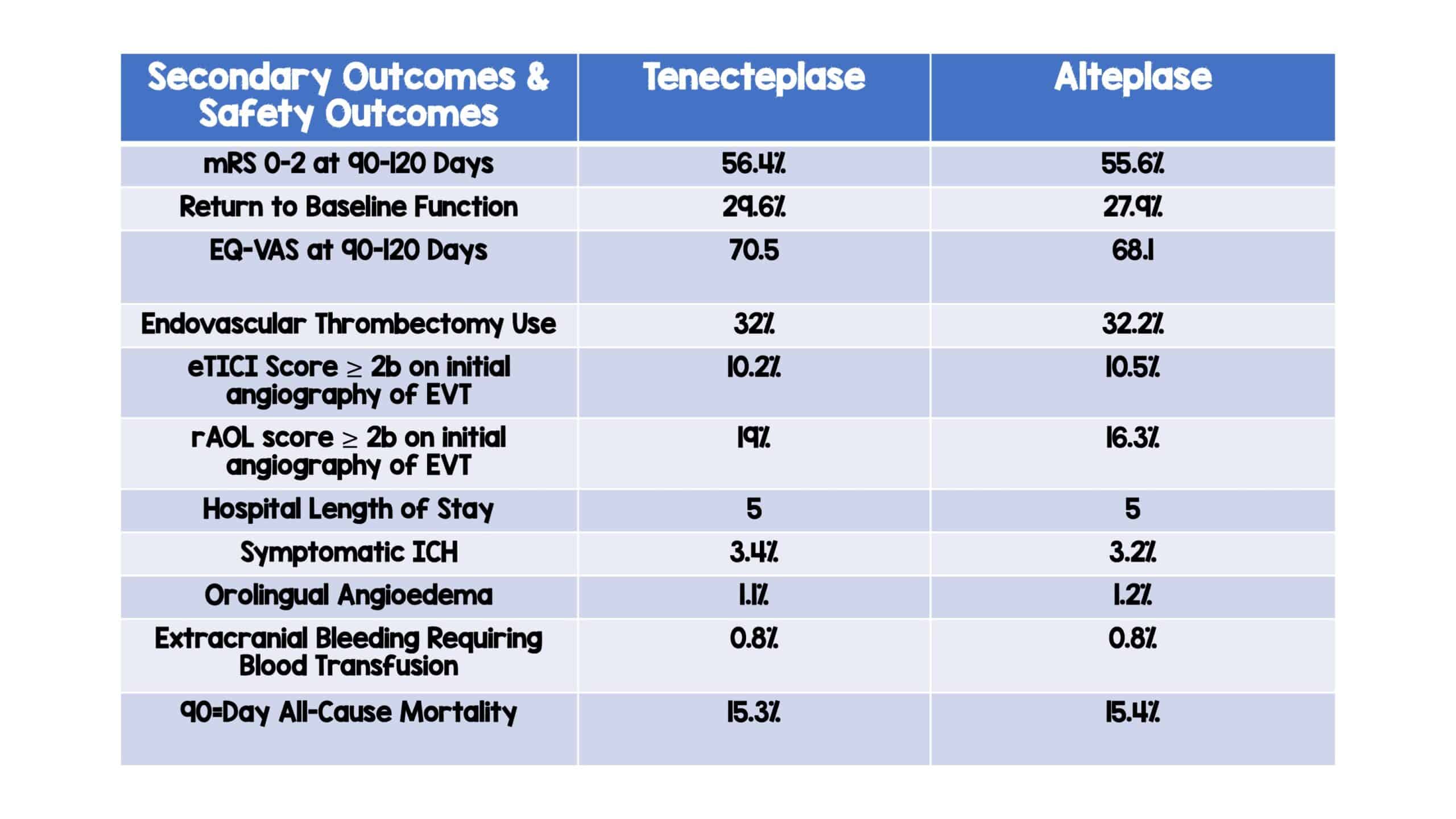
- Safety Outcomes:
- Symptomatic ICH: Tenecteplase (3.4%) vs Alteplase (3.2%)
- Orolingual angioedema: Tenecteplase (1.1%) vs Alteplase (1.2%)
- Extracranial bleeding requiring blood transfusion: Tenecteplase (0.8%) vs Alteplase (0.8%)
- 90-day all-cause mortality: Tenecteplase (15.3%) vs Alteplase (15.4%)
Strengths:
- This is the first phase 3 RCT to show that IV thrombolysis with tenecteplase (0.25 mg/kg) is comparable to alteplase in terms of efficacy and safety in patients with acute ischemic stroke presenting within 4.5h of symptom onset
- Multi-center (22 primary and comprehensive stroke centers), RCT with large sample size and pragmatic eligibility criteria, making the study more reflective of standard practice
- Non-inferiority trial design and relevant primary outcome that are patient centered
- Consistency of results across multiple secondary outcomes and subgroups increases believability and generalizability of trial results
- Baseline stroke severity distributions in this trial were similar between groups
- Adds further information to a question with inconsistent consensus
- Very low loss to follow-up
- The funders of this study had no role in study design, data collection, data analysis, data interpretations, or writing of the report.
Limitations:
- Open label study design
- Outcome assessments were done via telephone interview.
- Low proportion of patients from primary stroke centers due to the COVID-19 pandemic
- Inability to recruit consecutive patients at some sites due to the COVID-19 pandemic
- Broad definition of symptomatic intracranial hemorrhage
- Enrollment during daytime hours only at some sites
Discussion:
- In this non-inferiority trial, the investigators conclude that tenecteplase is non-inferior to alteplase, the current standard of care for eligible patients with AIS when looking at the primary outcome of mRS 0-1.
- The use of a non-inferiority design makes sense for this study question. When evaluating two things and looking for comparability, there has to be an added benefit of the newer medication. In this case, tenecteplase has a higher fibrin specificity, easier dosing and logistic implications, and a cheaper cost.
- The study is large, and the results of the study do help to fill a gap in the literature on tenecteplase for AIS. If you feel that alteplase works effectively in the setting of AIS, then it is reasonable to consider tenecteplase as an efficacious and safe alternative that allows for a more streamlined clinical flow.
- The big limitation is the open enrollment study design, which means that the study was not completely blinded. The treatment allocation was open-label, with blinded outcome assessments, but health personnel and patients were not blinded. Knowledge of treatment allocation, especially with the use of a scoring system that has inconsistent inter-rater reliability such as the mRS, may lead to significant detection bias.
- Another thing that was interesting was that mRS outcomes were assessed via telephone interviews. Again, comes back to the wide inter-rater reliability of mRS scores even among stroke experts examining a patient in front on them. Reliance on research coordinators to accurately determine mRS scores over a telephone interview could suffer from issues of reliability.
- Another interesting finding was that there were slightly more patients with a baseline NIHSS < 8 in the tenecteplase group (40.5%) compared to the alteplase group (38.4%). This may potentially bias the overall results of this study.
Author Conclusion: “Intravenous tenecteplase (0·25 mg/kg) is a reasonable alternative to alteplase for all patients presenting with acute ischemic stroke who meet standard criteria for thrombolysis.”
Clinical Take Home Point: In this study, the authors note that tenecteplase was non-inferior to alteplase in terms of functional outcomes at 90-120 days with no difference in safety outcomes and may have practical or real-world applications that make it more attractive compared to alteplase. Yet, this study has significant limitations and the fundamental comparison of tenecteplase to alteplase also remains an issue regarding robust evidence for thrombolytic efficacy to begin with. But, if a thrombolytic agent is to be given, tenecteplase would be a reasonable alternative to alteplase with similar efficacy, safety profile, and improved operational logistics. Additionally, more research and future studies are needed to show superiorly or reproduce these results in regard to thrombolytic agents.
References:
- Memon BK et. Intravenous tenecteplase compared with alteplase for acute ischemic stroke in Canada (AcT): a pragmatic, multicenter, open-label, registry-linked, randomized, controlled, non-inferiority trial. Lancet. 2022. PMID: 35779553
For More Thoughts on This Topic Checkout:
- REBEL EM: Tenecteplase vs Alteplase in Acute Ischemic Stroke
- REBEL EM: Tenecteplase for Thrombolysis of AIS
- REBEL EM: EXTEND-IA – TNK Part II
- REBEL EM: Tenecteplase vs Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK)
Post By:
Muhammad Durrani, DO
Assistant Clerkship Director, Assistant Research Director
Inspira Medical Center, Vineland NJ
Twitter/X: @ibbydurrani
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)





