Andexanet alfa (AA) is a decoy protein specific to the emergent reversal of Factor Xa inhibitor-associated bleeding. The ANNEXA-4 trial concluded that AA was an effective reversal agent in factor Xa-inhibitor-associated bleeding, with 82% of patients demonstrating excellent or good hemostasis at 12 hours. (Connolly 2019) However, the study did not compare the reversal agent to 4F-PCC, which raised questions about its safety and effectiveness. The question remains—Which reversal agent is ideal in anticoagulant-associated intracranial bleeding (ICB)?
Recent comparative studies have found no statistical difference in hemorrhage expansion or thromboembolic complications when either 4F-PCC or AA were used for factor-Xa inhibitor-associated-ICB. (Ammar 2021, Pham 2022, Parsel 2022) However, Korebey et al. sought to compare their data on 4F-PCC to the ANNEXA-4 trial. They concluded that 4F-PCC is equivalent to, if not superior, in outcomes when used for the reversal of factor Xa inhibitor associated-intracranial hemorrhage. (Korebey 2021) These studies were retrospective in design and are subject to inherent limitations. To date, there are no prospective or randomized studies comparing the effectiveness and safety of 4F-PCC and Andexanet alfa in ICB.
This study used propensity scoring-overlap weighting, a method that attempts to reproduce the statistical rigor of a randomized control trial, to analyze the safety and effectiveness of Andexanet alfa and 4F-PCC in patients with intracranial hemorrhage.
Article: Costa O.S. et al. Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhage: a propensity score-overlap weighted analysis. Crit Care 26, 180 (2022). PMID: 35710578
Clinical Question: Compared to 4F-PCC, is Andexanet-alfa safe and effective for the reversal of factor Xa inhibitor-associated intracranial hemorrhages?
WHAT THEY DID
- This is a two-cohort indirect comparative analysis of patients from the ANNEXA-4 trial and an observational synthetic control arm of 4F-PCC patients.
- The synthetic control arm was obtained retrospectively from one of three acute care hospitals in the Hartford Healthcare network between 12/1/2016 and 8/30/2020.
- All patients were identified as having an intracranial hemorrhage on initial CT evaluation and recent apixaban or rivaroxaban use within the last 24 hours.
- An interval CT scan at approximately 12 hours post-reversal administration was obtained and reviewed for hemorrhage stability.
- A propensity scoring-overlap weighting approach was utilized to match patients in both cohorts and account for confounding variables.
POPULATION
Inclusions:
- Age ≥ 18 years of age
- Radiographically confirmed acute spontaneous or traumatic intracranial hemorrhage (i.e. subdural, subarachnoid, and intraparenchymal).
- Factor-Xa inhibitor (apixaban or rivaroxaban) use within the past 24 hours
- ANNEXA-4 study
- Participants within the US
- Synthetic control arm
- Multi-center participants within the Hartford Healthcare network
Exclusions:
- Glasgow Coma Scale < 7 upon admission
- Intracerebral bleed volume > 60ml
- Planned surgery within 12 hours from the initial scan
INTERVENTION
- 96.6% of patients received 400 mg bolus + 440 mg infusion of AA
COMPARATOR
- Out of 95 patients in the 4F-PCC cohort, 79.3% (75 patients) received 25 unit/kg dose.
OUTCOMES
Primary:
- Hemostatic efficacy (excellent/good vs. poor/none)
- Excellent/good hemostatic efficacy was defined as a ≤ 35% increase in hemorrhage expansion seen on interval CT-head imaging compared to the initial.
- Volume was calculated for intracerebral bleeds using the abc/2 method, and thickness was measured for subarachnoid and subdural hemorrhages.
- 30-day all-cause mortality
Secondary:
- Thromboembolic events within the first 5 days of 4F-PCC or AA administration.
RESULTS
- Study participants in the ANNEXA-4 trial.
- 477 patients were enrolled in the trial
- 370 patients were excluded
- 107 patients were reviewed.
- Study participants from one of three Hartford Healthcare hospitals.
- 385 patients with intracranial hemorrhages were enrolled
- 290 patients were excluded, and 95 patients were retrospectively reviewed after receiving 4F-PCC.
- Before propensity matching…
- AA patients were more likely to be concomitantly on antiplatelet medication compared to 4F-PCC; (33.6% vs. 24.2%).
- A greater percentage of AA patients presented with large hemorrhage sizes (≥ 10ml/mm) compared to 4F-PCC; (33.6% vs. 14.7%).
- A lower dose of the reversal agent was used more frequently in both cohorts (96% in AA and 74.3% in 4F-PCC).
- After propensity matching…
- The mean time from the initial scan to the anti-coagulation reversal initiation was 2.3 hours.
- The mean time from reversal agent administration to repeat scan was 12.2 hours.
- Atrial fibrillation was the primary indication for oral anticoagulation (86.4%)
- Traumatic bleeds (61.1%)
- Hemostatic Effectiveness
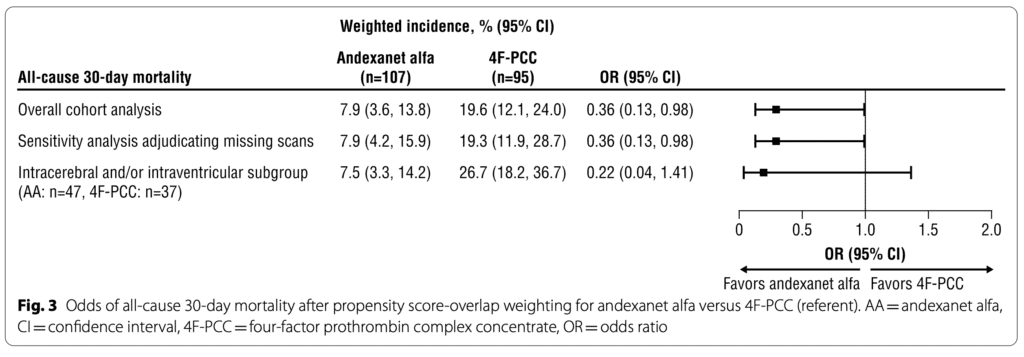
- Excellent/good hemostatic effectiveness was more significant in the AA group compared to the 4F-PCC; (85.8% vs. 68.1%) [OR of 2.73 (95% CI 1.16-6.42)].
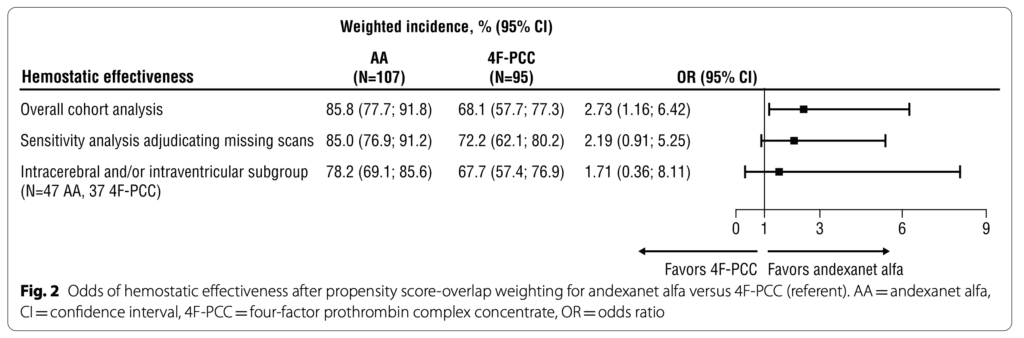
- All-cause mortality
- Before propensity score matching, there was a higher incidence of mortality secondary to worsening intra-cranial hemorrhage amongst the 4F-PCC group (50%) compared to the AA group (20%).
- There were two thromboembolic events reported in the AA group within five days compared to zero events in the 4F-PCC group.
STRENGTHS
- The 4F-PCC arm study design mimicked that of ANNEXA-4 to reduce bias.
- Propensity score overlap weighting addressed concerns for confounders making this study compared to a randomized control trial.
- After propensity matching, baseline characteristics and hemorrhage characteristics were similar.
- There was a comparison between hemostatic effectiveness, mortality, and thrombotic complications.
- This was a clinically relevant research question.
- Investigators performed a sensitivity analysis with adjudication of outcomes from 8 patients in the 4F-PCC group with missing information.
LIMITATIONS
- This is the largest study of its kind at the publication date, but still, this is a relatively small study (N= < 200), which limits the external validity.
- This is not a direct comparison or head-to-head trial. Investigators compared AA from an open-label multicenter trial with no control to a synthetic observational cohort from a different healthcare system.
- Do patients in the AA arm carry the same risk as patients in the 4F-PCC arm?
- Each cohort has distinct limitations.
- The findings of the ANNEXA-4 trial have been met with skepticism
- The open-label study design and lack of a comparator in the AA arm introduce bias.
- The observational nature of the synthetic cohort can only show association and not causation.
- Investigators excluded patients who were very ill and likely to die from bleeding. Given the natural history of the disease process, patients may have had similar outcomes without treatment.
- The primary outcomes were disease-oriented and not-patient-oriented.
- Functional outcomes were not discussed or reported.
- In the ANNEXA-4 trial, patients were actively surveyed for thromboembolic events, while those in the 4F-PCC arm were likely not.
- Approximately 75% of patients in the 4F-PCC cohort received a lower dose despite some guidelines recommending a higher dose.
- Residual confounding of unobserved or unmeasured covariates may exist despite propensity score-overlap weighting.
- The study was funded, written, and formally reviewed by Alexion, AstraZeneca—makers of AA.
DISCUSSION
REBEL Rant
- This is not an RCT… It appears to be more bad science hidden behind the complex statistical methodology. Comparing the US cohort of patients from the ANNEXA-4 trial with a synthetic observational cohort is problematic. The ANNEXA-4 trial has several serious and heavily debated methodologic concerns (see “For more information” section” below). Investigators in this paper did not perform a direct comparison, and we have zero information about other interventions for patients in the synthetic cohort 4F-PCC arm. In an open-label trial, it’s possible patients in the AA arm were treated more aggressively outside the study protocol.
- The primary outcome —hemostatic effectiveness— is disease-oriented. Investigators also looked at all-cause mortality but they did not attempt to investigate functional status or neurologic outcomes. Will patients care if they have a ≤ 35% increase in hemorrhage expansion if they are severely disabled or in a vegetative state?
- Propensity Score with overlap weighting is sold as a method that “mimics” an RCT in observational trials when an RCT is not possible. But it remains unclear if AA is superior to 4FPCC. There is also no clear reason why an RCT cannot be performed, and in fact, a large RCT is underway.
- There are serious conflicts of interest in this paper. The study was funded, written, and formally reviewed by Alexion, AstraZeneca—makers of AA. This publication appears to be just another attempt to sell a product.
Inside the Numbers
- The investigators report that hemostatic effectiveness is 2.7 times more likely to occur in the presence of AA use compared to 4F-PCC use in the setting of Factor Xa- inhibitor-associated ICB. However, on sensitivity analysis, the 95% confidence intervals for OR cross 1. In addition, the AA cohort was ⅓ less likely to die from any cause compared to 4F-PCC use in the setting of Factor Xa- inhibitor-associated ICB. The upper limit of 95% CI nearly crossed 1 (OR 0.36 [0.13–0.98]). Ultimately, The small sample sizes and wide confidence intervals make us skeptical of the findings.
Propensity Score with Overlap Weighting
- The propensity score is the probability that a patient will receive a treatment based on their measured covariables. A propensity score is calculated for every patient in the trial.
- Overlap weighting is a method that allows for the comparison of two different cohorts. In this study patients who received an AA in the ANNEXA-4 trial and the separate cohort who received 4F-PCC.
- This method is used to compare two groups in an observational study when an RCT is not feasible.
- Performing an RCT is difficult in this treatment group as withholding a “potentially” life-saving treatment maybe considered unethical.
Author’s Conclusion: “Our indirect comparison analysis of ANNEXA-4 derived Andexanet alfa patients and a synthetic control arm of 4F-PCC patients from a US healthcare system showed that Andexanet alfa was associated with better hemostatic effectiveness and reduced odds of all-cause mortality at 30 days. Our findings support current consensus guidelines, which preferentially recommend using Andexanet alfa over 4F-PCC for the management of apixaban- or rivaroxaban-associated life-threatening bleeds, including intracranial hemorrhage.”
Our Conclusion:
The numerous methodologic concerns and conflicts of interest in this trial and the parent (ANNEXXA-4) trial make us wary about the data presented. Until large, externally validated, randomized controlled trials prove otherwise, we remain skeptical about the efficacy of Andeaxanet alfa for managing apixaban- or rivaroxaban-associated life-threatening bleeds, including intracranial hemorrhage.
References:
- Xian Y, Zhang S, Inohara T, et al. Clinical Characteristics and Outcomes Associated With Oral Anticoagulant Use Among Patients Hospitalized With Intracerebral Hemorrhage. JAMA Netw Open. 2021;4(2):e2037438. PMID: 33591368
- Connolly, S. J., Crowther, M., Eikelboom, J. W., Gibson, C. M., Curnutte, J. T., Lawrence, J. H., Yue, P., Bronson, M. D., Lu, G., Conley, P. B., Verhamme, P., Schmidt, J., Middeldorp, S., Cohen, A. T., Beyer-Westendorf, J., Albaladejo, P., Lopez-Sendon, J., Demchuk, A. M., Pallin, D. J., . . . Milling, T. J. (2019, April 4). Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. New England Journal of Medicine, 380(14), 1326–1335. PMID: 30730782
- Ammar AA, Ammar MA, Owusu KA, Brown SC, Kaddouh F, Elsamadicy AA, Acosta JN, Falcone GJ. Andexanet Alfa Versus 4-Factor Prothrombin Complex Concentrate for Reversal of Factor Xa Inhibitors in Intracranial Hemorrhage. Neurocrit Care. 2021 Aug;35(1):255-261. Epub 2021 Jan 6. PMID: 33403588
- Pham H, Medford WG, Horst S, Levesque M, Ragoonanan D, Price C, Colbassani H, Piper K, Chastain K. Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhages. Am J Emerg Med. 2022 May;55:38-44. PMID: 35272069
- Parsels KA, Seabury RW, Zyck S, Miller CD, Krishnamurthy S, Darko W, Probst LA, Latorre JG, Cwikla GM, Feldman EA. Andexanet alfa effectiveness and safety versus four-factor prothrombin complex concentrate (4F-PCC) in intracranial hemorrhage while on apixaban or rivaroxaban: A single-center, retrospective, matched cohort analysis. Am J Emerg Med. 2022 May;55:16-19. PMID: 35245776
- Korobey MJ, Sadaka F, Javed M, Moynihan M, Alsaei A. Efficacy of 4-Factor Prothrombin Complex Concentrates in Factor Xa Inhibitor-Associated Intracranial Bleeding. Neurocrit Care. 2021 Feb;34(1):112-120. PMID: 32430804
For More Thoughts on This Topic
- First10EM: Andexanet Alfa: More Garbage Science in the NEJM
Guest Post By:

Carlton C.L. Watson, MD, MSc
PGY-1 , Emergency Medicine Resident
Nuvance Health, Poughkeepsie, New York
Twitter: @justcarlton

Marco Propersi, DO, FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)