Article: Bramante CT et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a Multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis 2023 PMID: 37302406
Clinical Question: Does outpatient treatment with ivermectin, fluvoxamine or metformin soon after SARS-CoV-2 infection reduce the risk of developing long-COVID in overweight patients?
Population: Adults aged 30-85 years of age with BMI > 25 (> 23 in those identifying as Asian or Latino) who had COVID-19 symptoms for fewer than 7 days and a documented SARS-CoV-2 positive PCR or antigen test within 3 days of enrollment without prior SARS-CoV-2 infection. Participants were recruited remotely through online advertising, patient portal messages and health-system advertising. Prior vaccination was not an exclusion criteria.
Outcomes:
- Primary: The development of long-COVID up to 300 days after enrollment. This secondary outcome became the primary outcome (see discussion for more detail).
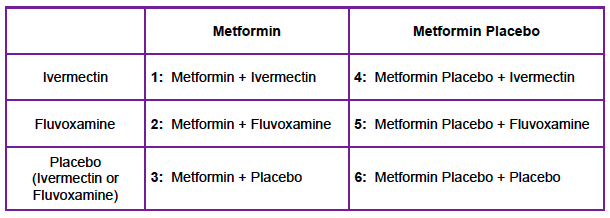
Intervention: 2 x 3 factorial design (see table below)
- Metformin 500mg qD on day 1, 500mg BID on days 2 to 5, then 500mg qAM and 100mg qHS up to day 14
- Ivermectin 390 to 470ug/kg/d x3d
- Fluvoxamine 50mg qD x1d followed by 50mg BID up to day 14
Control: Identical placebo pills
Design: Multicenter, randomized, quadruple-blinded, parallel group, phase 3 study.
Excluded:
- Patients already taking one of the study medications
Primary Results
-
- Enrolled: 1431 patients
- 1126/1323 participants in the modified, intention-to-treat population were consented for long-term follow up and completed at least one survey.
- Metformin: 564 patients
- Placebo: 562 patients
- Median BMI ≈30kg/m2
- Vaccination Status
- SARS-CoV-2 Primary Vaccination ≈55% of enrolled patients
- SARS-CoV-2 Booster Vaccination ≈5% of enrolled patients
- Median time from symptom onset to study drug initiation =5d
- SARS-CoV-2 Dominant Variant at Time of Randomization was the Delta Variant in 70% of patients
- Enrolled: 1431 patients
Critical Findings:
- 93/1126 (8.3%) reported a long COVID diagnosis by day 300.
- Alpha dominant: 7.9%
- Delta dominant 8.3%
- Omicron dominant: 8.4%
- No statistically significant differences found for fluvoxamine or ivermectin.
|
Metformin (95% CI) |
Placebo (95% CI) |
Absolute Difference |
Hazard Ratio (95% CI) |
NNT |
||
|
Long COVID |
6.3% (4.2-8.2) |
10.4% (7.8-12.9) |
4.1% |
0.59 (0.39 – 0.89) |
25 |
Statistically Significant Difference |
Strengths:
- The study asks an important, patient centered question.
- Broad inclusion criteria increasing external validity.
- Pregnant/lactating patients were not excluded.
- Blinding procedures were well constructed as to mask group allocation.
- Robust patient follow-up process tailored to the patient’s preference.
- Had a very precise four-part definition of severe COVID-19 (Hypoxemia at home, ED Visit, Hospitalization, death)
- 95% of participants completed at least 9 months of follow-up
- Patient had to receive diagnosis of long-COVID from a medical professional (not simply based on symptom reporting).
Limitations:
- Enrollment was investigator initiated which may introduce bias.
- Relatively small study given the scope of the disease.
- This trial excluded groups at low risk of severe COVID-19—adults with a normal BMI and those who were younger than 30 years—and whether these findings would be generalisable to those populations remains unknown
Discussion
- This is the first, high-quality study showing that the incidence of long-COVID can be reduced through a medical intervention.
- This study helps to solidify the diagnosis of long COVID; for an effective therapy to exist, there must also exist a disease (see Faust editorial linked below).
- The original trial investigated the rate of severe COVID-19 by day 14. As a secondary outcome, the authors wanted to investigate the development of long COVID.
- On the face of it, this appears to be publication of a secondary outcome and the authors state as much.
- However, it is slightly more complicated: When the authors became aware of the phenomenon of long COVID, they reconsented patients into this separate study prior to any analysis of data or unblinding.
- Essentially, the authors adapted to a rapidly evolving situation in order to get the best possible data to influence decisions.
- The mechanism of action for metformin’s reduction in long-COVID is not well-understood. More research is necessary in this area.
- This data does not speak to whether metformin can be given after the development of long-COVID in efforts to ameliorate the disease. Future research in this area is necessary.
- This study provides additional data that vaccination reduces the risk of developing long-COVID. Among participants who had received at least the primary SARS-CoV-2 vaccine series, 41 (6.6%) of 619 reported a diagnosis of long COVID, compared with 52 (10.3%) of 507 who were unvaccinated.
Authors Conclusions: “Outpatient treatment with metformin reduced long COVID incidence by about 41%, with an absolute reduction of 4·1%, compared with placebo. Metformin has clinical benefits when used as outpatient treatment for COVID-19 and is globally available, low-cost, and safe. “
Our Conclusions: We agree with the authors. A short course of metformin reduces the risk of long COVID incidence in overweight/obese adults 30-85 years of age when started early in the course of clinical COVID-19.
Potential to Impact Current Practice: With the potential of hundreds of millions of people to be diagnosed with long COVID in the absence of treatment, metformin could have a significant impact without a large price. Just as important, this study helps to solidify long COVID as a true entity.
Bottom Line: A short course of metformin should be considered in overweight/obese patients with COVID-19 who present early in their disease course to reduce the risk of developing long COVID.
References:
- Bramante CT et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a Multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis 2023 PMID: 37302406
- Faust J. The therapeutic validation of long-COVID. Lancet Infect Dis 2023. PMC: 10250006
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie) and Mark Ramzy, DO (Twitter: @MRamzyDO)