Paper: Janiaud P et al. Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-Analysis. JAMA 2021. PMID: 33635310
Clinical Question: Is treatment with convalescent plasma associated with improved clinical outcomes in patients with COVID-19?
What They Did:
- Systematic review and meta-analysis of clinical outcomes with convalescent plasma therapy vs placebo or standard of care in peer-reviewed and preprint publications or press releases of randomized clinical trials
- Searched PubMed, Cochrane COVID-19 trial registry, and the Living Overview of Evidence platforms
- Primary Analysis: Peer-reviewed publications of RCTs only
- Secondary Analysis: All publicly available RCTs (peer reviewed, preprints, and press releases)
Outcomes:
- All-cause mortality at any time point
- Length of hospital stay
- Clinical improvement
- Clinical deterioration
- Mechanical ventilation use
- Serious adverse events
Inclusion:
- RCTs (Peer-reviewed, preprints, and press releases) that had:
- Suspected or confirmed SARS-CoV-2 infection
- Randomly allocated to receive convalescent plasm versus placebo together with standard of care or only standard of care
- Included trials regardless of level of plasma titer (low or high antibody titer) or health care setting
Exclusion:
- RCTs aimed at preventing occurrence of COVID-19
Results:
- Primary Analysis: 1060 patients from 4 peer reviewed RCTs
- Secondary Analysis: 10,722 patients from 6 other publicly available RCTs
- Location of RCTs:
- 3 conducted in India
- 2 conducted in Argentina
- 1 conducted in Bahrain, China, Netherlands, Spain, and the UK
- Termination of RCTs:
- 5 RCTs were terminated early
- 2 due to futility
- 3 due to slow recruitment
- 5 RCTs were terminated early
- Blinded RCTs:
- 2 RCTs were double-blind
- 8 RCTs were open label (unblinded)
- Doses of Convalescent Plasma Given in RCTs:
- 5 of the RCTs administered a single dose of plasma
- 5 of the RCTs administered two doses of plasma
- Titers of Convalescent Plasma Used in RCTs:
- 4 RCTs used high plasma titer
- 1 RCT used low titer
- 3 RCTs did not use a minimum plasma titer cutoff
- 2 RCTs it was unclear what titer was used
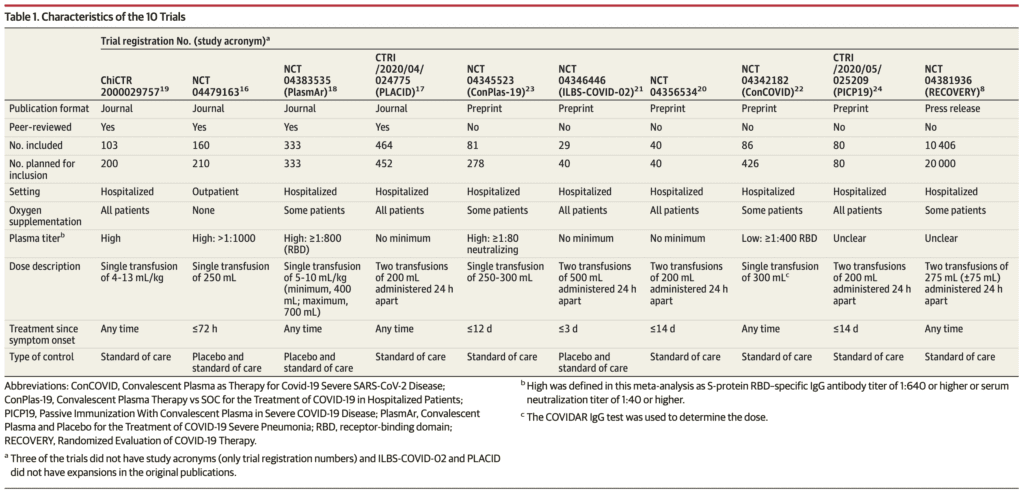
- Risk of Bias:
- Risk of bias was deemed low for 7 of the 10 RCTs on the outcomes of mortality, length of hospital stay, and mechanical ventilation
- Loss to follow up was <10% in 9 of the 10 RCTs (Data unavailable for RECOVERY Trial)
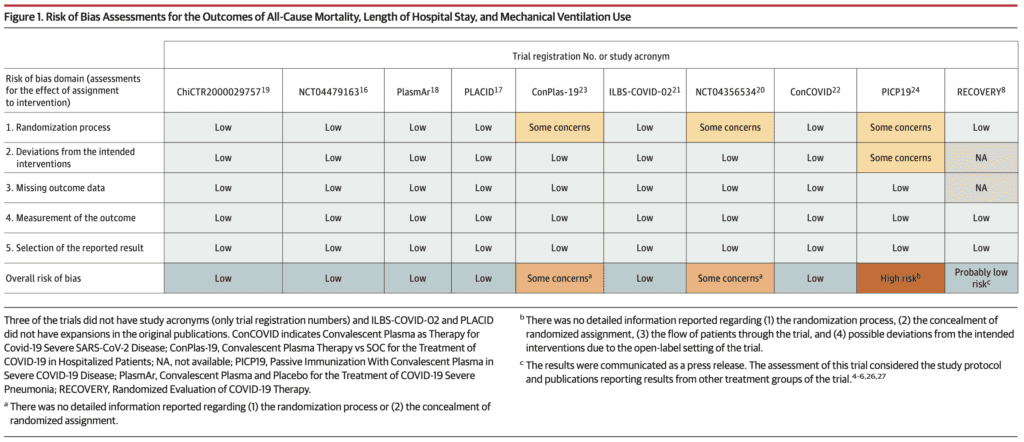
- All-Cause Mortality (10 of 10 RCTs):
- 8 of 10 trials assessed between 15 to 30d after randomization
- Primary Analysis: RR 0.93; 95% CI 0.63 to 1.38
- CPT: 11.6%
- Control: 12.7%
- Secondary Analysis: RR 1.02; 95% CI 0.92 to 1.12
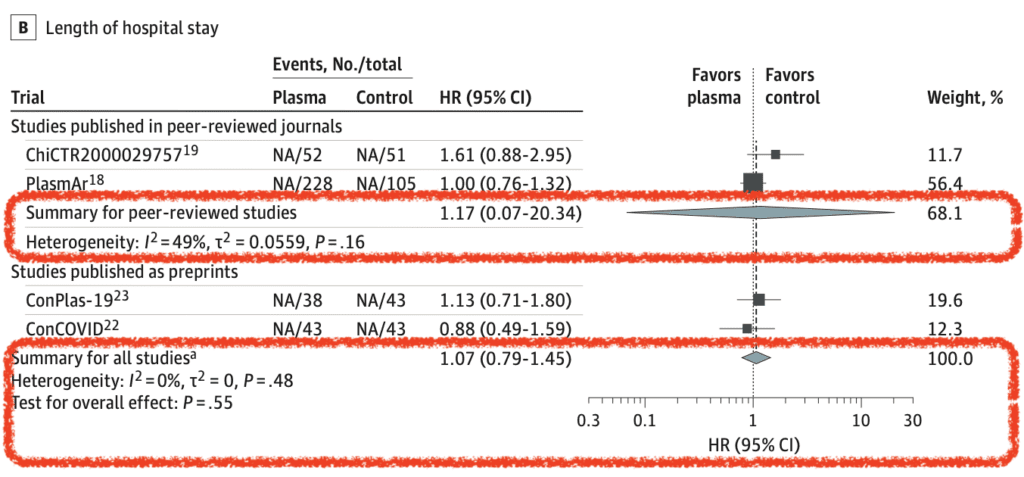
- Length of Hospital Stay (7 of 10 RCTs):
- Primary Analysis: HR 1.17; 95% CI 0.07 to 20.34
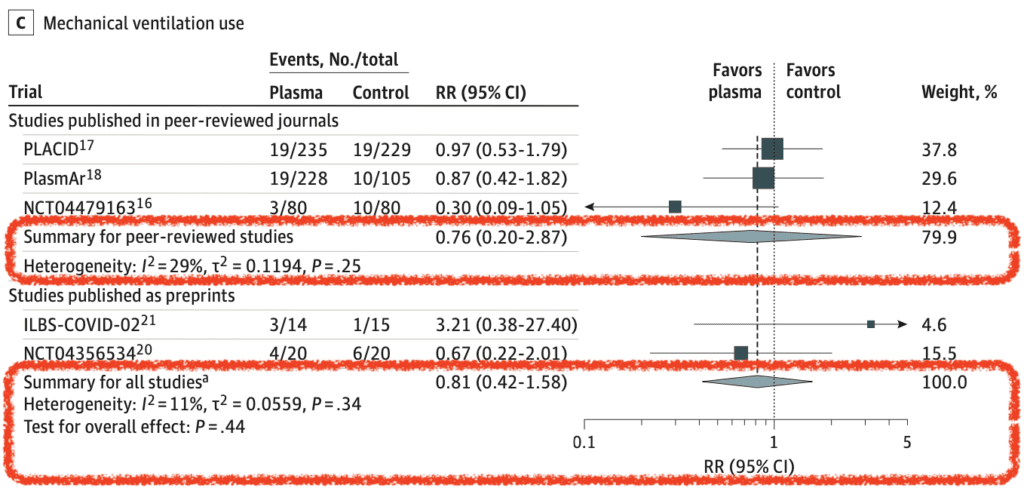
- Mechanical Ventilation Use (5 of 10 RCTs):
- Primary Analysis: RR 0.76; 95% CI 0.20 to 2.87
- Limited data on clinical improvement, clinical deterioration, and serious adverse events showed no significant differences
Strengths:
- Followed the Preferred Reporting Items for Systematic Review and Mata-Analysis (PRISMA) guidelines
- Included only peer-reviewed RCTs as primary outcome and additionally non-peer-reviewed publications as a secondary outcome in order to look at the totality of available evidence
- Two reviewers independently assessed the risk of bias for all-cause mortality, mechanical ventilation use, and length of hospital stay
- Used the Cochrane Risk of Bias Assessment Tool to identify risk of bias
- Also used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to assess the certainty of the evidence for the summarized outcomes
- Performed multiple sensitivity analyses to assess the robustness of the results
Limitations:
- Treatment effects for clinical improvement, clinical deterioration, and serious adverse events were not summarized due to inconsistent definitions of these outcomes and insufficient reporting of relevant details
- For the outcome of mortality, the RECOVERY Trial which was a press release accounted for 90.2% of the weight and 88.3% of the patients. The results therefore should warrant cautious interpretation until the trial results are fully analyzed
Discussion:
- For the primary analysis that only included the 4RCTs published in peer-reviewed journals the certainty of evidence (using GRADE) for mortality was low with wide 95% CIs. For the secondary analysis that included all 10RCTs published the concern regarding imprecision was reduced and the certainty of evidence was rated as moderate
- For length of hospital stay and mechanical ventilation use, the certainty of the evidence was rated as low for peer reviewed trials only, and the addition of all publicly available trials did not change this rating due to wide 95% Cis
- Variation in outcome measures across trials makes precise conclusions a bit more challenging
- 3 of the 10 RCTs had some concerns or high risk of bias, but only accounted for 1.8% of the weight of the meta-analysis on all-cause mortality
- Subgroup analyses were unable to be performed due to limited individual patient level data
- Only 1 RCT was in outpatients and therefore the conclusions of this summary should not be extrapolated to this patient population (i.e. Mild COVID-19)
Author Conclusion: “Treatment with convalescent plasma compared with placebo or standard of care was not significantly associated with a decrease in all-cause mortality or with any benefit for other clinical outcomes. The certainty of the evidence was low to moderate for all-cause mortality and low for other outcomes.”
Clinical Take Home Point: This is a very well-done systematic review and meta-analysis of the available evidence on the use of convalescent plasma therapy compared to placebo or standard of care. Based on this review, convalescent plasma therapy is not associated with a decrease in all-cause mortality or with any benefit for other clinical outcomes among patients with COVID-19 and should not be standard therapy. Just because it makes sense doesn’t mean it will work. This review is further evidence that high-level evidence is required to help guide care.
References:
- Janiaud P et al. Association of Convalescent Plasma Treatment With Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-Analysis. JAMA 2021. PMID: 33635310
- RECOVERY Trial. Press Release: RECOVERY Trial Closes Recruitment for Patients Hospitalised with COVID-19. January 2021. Accessed March 2nd, 2021. [Link is HERE]
For More Thoughts on This Topic Checkout:
- St. Emlyn’s Blog: Convalescent Plasma (Still) Does Not Work in COVID-19
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)



