Paper: Joyner MJ et al. Effect of Convalescent Plasma on Mortality Among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv 2020. [Epub Ahead of Print]
Clinical Questions:
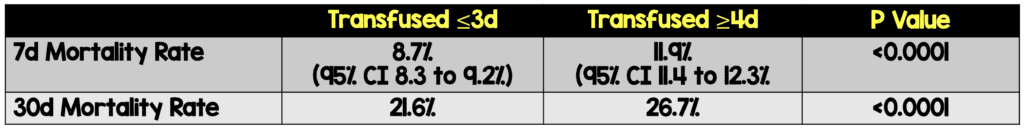
- Is CPT earlier (≤3 days from symptom onset) vs later (≥4 days from symptom onset) associated with reduced morality among hospitalized patients with COVID-19?
- Are higher CPT antibody levels compared to lower antibody levels associated with reduced mortality among hospitalized patients with COVID-19?
What They Did:
- Multicenter, open-label, expanded access program (EAP) for the treatment of COVID-19 patients
- CPT collected from patients after being symptom free for 14 days
- Compatible COVID-19 CPT was administered IV. Enrolled patients had to have at least one unit (≈200mL) administered with the option to administer additional doses if clinically justified
- Antibody testing was based on the sample signal-to-cut-off (S/Co) ratio
- Low Level = <4.62 S/Co
- Medium Level = 4.62 to 18.45 S/Co
- High Level = >18.45 S/Co
- Cohorts stratified into categories based on days from COVID-19 diagnosis to plasma transfusion (0, 1 – 3, 4 – 10, and ≥11d)
Outcomes:
- Primary: 7 and 30d mortality
- Secondary: Safety of transfusion of COVID-19 CPT
Inclusion:
- Age ≥18 years
- Hospitalized with laboratory confirmed SARS-CoV-2 infection
- Judged to be at high risk of progression to severe or life-threatening COVID-19
- Definition of severe or life-threatening:
- Dyspnea
- RR ≥30BPM
- Blood O2 sat ≤93%
- P/F ratio <300
- Lung infiltrates >50% within 24 to 48hrs
- Respiratory failure
- Septic shock
- Multiple organ failure
- Definition of severe or life-threatening:
Exclusion:
- None
Results:
- > 47k patients enrolled in EAP
- 36,226 patients were transfused with CPT
- Data included for 35,322 transfused patients
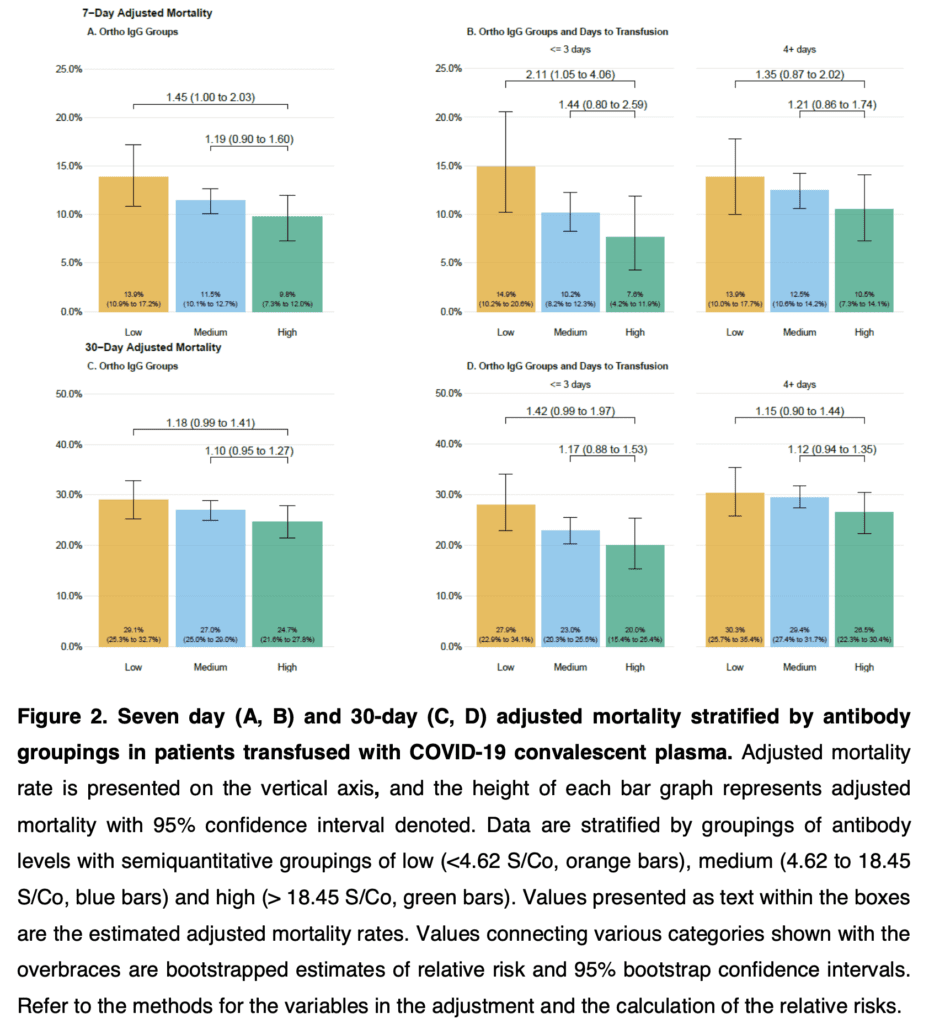
- 7- and 30-Day Mortality Rate Based on IgG Antibody Levels in Convalescent Plasma:
- Gradient of mortality decrease from low > medium > high IgG levels
Strengths:
- Used two different analytical methods to control for confounding
- Analyzed data from 1809 sties and >35,000 patients with COVID-19
- Used historical data of CPT in respiratory illnesses to determine early vs late treatment
- Study designed to provide access to CPT at hospitals and acute care facilities that did not have infrastructure to support RCTs
- Degree of immune activity within units of CPT was not known at the time of administration. Not knowing low, medium and high antibody levels at time of treatment creates a pseudo-randomization
Limitations:
- Non-randomized trial containing multiple sources of possible confounding (i.e. concomitant medications, plasma volume, non-uniform antibody levels between multiple plasma units, etc…)
- No placebo group to compare treatment with no treatment on mortality rates
- Which medications patients should be on to participate was not pre-specified nor randomized
- 11k patients who were enrolled were not given CPT which can create a selection bias
- Data was not analyzed for >1000 patients that did get CPT
- No discussion of whether this was consecutive enrollment
- Only administered CPT in the critically ill subgroup
Discussion:
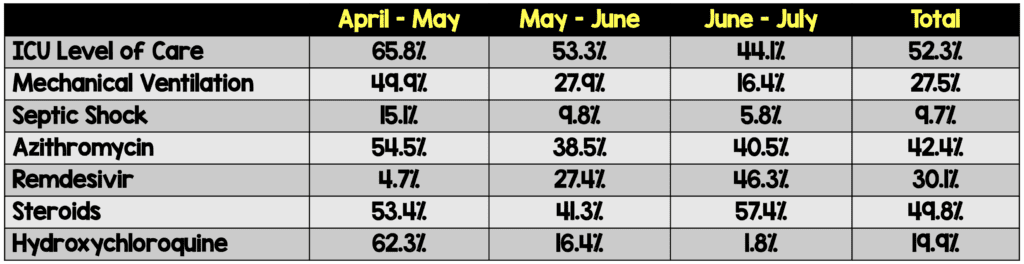
- Patients transfused early in the study period (before May 1st) were more critically ill (higher rates of mechanical ventilation, ICU admissions, and septic shock), had higher concomitant treatment with hydroxychloroquine and azithromycin, and lower concomitant treatment with remdesivir compared to groups transfused later in the study
- There is no data on the use of CPT in patients with mild/moderate illness and the results of this trial cannot be extrapolated to that group
- Without a placebo group we don’t truly know if CPT benefits patients compared to standard care alone
- Both the Open-label (everyone aware of who is getting what treatment) nature and Hawthorne effect (alteration of behavior due to awareness of being observed) play huge implications in this trial. Both of these factors could potentially make results look better than they are
- CPT is not a benign therapy. Working up in the ICU for the past couple of months I have seen a handful of Transfusion Related Acute Lung Injury (TRALI) patients after CPT. Although not really flushed out in this paper, it is important to remember this complication (this can occur in about 1 in 7,900 units of FFP) [2]
Author Conclusion: “The relationships between reduced mortality and both earlier time to transfusion and higher antibody levels provide signatures of efficacy for convalescent plasma in the treatment of hospitalized COVID-19 patients. This information may be informative for the treatment of COVID-19 and design of randomized clinical trials involving convalescent plasma.”
Clinical Take Home Point:
- Earlier use of CPT (≤3d) was associated with lower observed rates of 7- and 30-day mortality compared to later use of CPT (≥4d). However, without a standard care arm, there is no way to know if CPT is better than standard management
- Use of CPT with higher antibody levels was associated with reduced 7- and 30-day mortality
- There was a clear shift over 3 months in the treatment of COVID-19 in terms of:
- Mechanical ventilation: More mechanically ventilated early in pandemic
- Concomitant treatment with hydroxychloroquine/azithromycin: More use early in the pandemic
- Concomitant treatment with Remdesivir: More use later in the pandemic
References:
- Joyner MJ et al. Effect of Convalescent Plasma on Mortality Among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv 2020. [Epub Ahead of Print]
- Toy P et al. TRALI – Definition, Mechanisms, Incidence, and Clinical Relevance. Best Pract Res Clin Anaesthesiol 2009. PMID: 17650771
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)