There have been some animal and human studies that have advocated for early norepinephrine administration in septic shock improving hemodynamics and mortality. The issue, with these trials is that they were retrospective which means these studies suffer from the limitations of this type of methodology (i.e. convenience sampling, recall bias, confounding, and ultimately cannot determine causation, only association).
What They Did:
- A phase II, prospective, single center, randomized, double-blind, placebo-controlled clinical trial evaluating the use of early low-dose norepinephrine (eNE) vs standard care (SC) (i.e placebo) in adults with septic shock to increase shock control by 6 hours in Bangkok, Thailand
- eNE = 4mg of norepinephrine mixed in 250mL of D5W = 16ug/mL
- SC (Placebo) = 250mL of D5W
- Study drug infused via either peripheral line or central venous catheter (when available) at a starting rate of 0.05ug/kg/min without further titration
Outcomes:
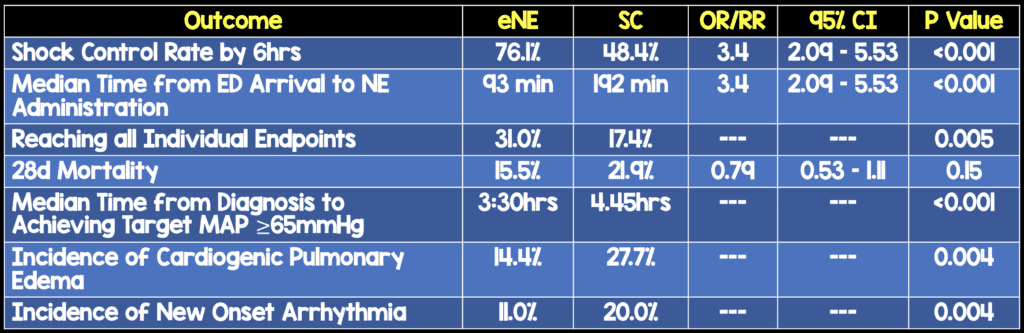
- Primary: Shock control rate (Defined as: achievement of MAP ≥65mmHg, with urine output ≥0.5mL/kg/hr for 2 consecutive hours OR decreased serum lactate ≥10% for baseline) by 6 hours after diagnosis
- Secondary:
- 28d Mortality
- Hospital Mortality
- Rate of respiratory failure requiring mechanical ventilator support
- Rate of renal failure requiring RRT
- Number of organ support-free days to day 28
- Safety:
- New onset of cardiac arrhythmia
- Organ ischemia
- Cardiogenic or non-cardiogenic pulmonary edema
Inclusion:
- Adults ≥18 years presenting to the ED with suspected infection and hypotension (MAP <65mmHg)
Exclusion:
- Meeting septic shock criteria for >1hr before randomization
- Acute CVA
- ACS
- GIB
- Acute pulmonary edema
- Status asthmaticus
- Active cardiac arrhythmias
- Pregnancy
- Seizure
- Drug overdose
- Burn injury
- Trauma
- Immediate surgery requirement
- Advanced stage cancer
Results:
- 310 adults diagnosed with septic shock
- No difference in number of patients admitted to ICU, amount of IV fluid received between groups
Strengths:
- First prospective RCT to investigate effect of early norepinephrine
- Randomization method was robust making it unlikely bias was introduced
- Treatment beyond the intervention was well balanced, including the addition of a pressor
- Patients balanced in background characteristics and disease severity
- Outcome evaluation, data management, and analysis were conducted by the principal investigator and a statistician, both of which were blinded to patient enrollment and treatment
- Patients, patients’ relatives, attending physicians, and nurses were all blinded to study assignment
- Pharmacist prepared study drug (norepinephrine or placebo) who had no other role in the trial
- Study drugs were packaged in identically shaped containers
- Patients all received infusion of crystalloid, appropriate antibiotic therapy, source control, and organ support
Limitations:
- Surrogate, short-term shock control endpoints without patient-oriented outcomes
- No objective measurements of altered perfusion to the gut and kidney were performed
- Patients may not have been “that” sick (i.e. APACHE scores reasonable but mean starting MAP = 56 mmHg)
- No patients required emergency intubation, although it is arguable if this matters or not
- The effects of norepinephrine could not be masked, which could have provided clues to physicians about which agent was being used
- 47% of patients transferred to medical floor which has different nursing to patient ratios (i.e. 1:3 vs 1:1)
- Mortality outcomes cannot be evaluated from the results of this study as this was not the aim of the trial
- There was no control on the resuscitation fluid rate
- Single center trial, limiting generalizability of findings to other care settings
Discussion:
- The timing of intervention in this study as a median of 93 minutes. Just for comparison the median time to intervention in ProCESS, ARISE, and PROMISE was 162 minutes in the EGDT arm and 159 minutes in the standard care arm
- The median fluids given before open-label norepinephrine was 2080mL vs 1900mL between groups and not statistically significant
- Although, secondary outcome of mortality was not statistically significant, it was clearly better in the eNE arm.
- Most if not all secondary endpoints were better in the eNE arm, but not statistically significant. As these are secondary endpoints, this would be hypothesis generating
- The authors of this paper appropriately state: “A multicenter trial with a larger population size, control of the rate of fluid resuscitation, and timing of norepinephrine initiation is certainly required to assess the survival benefit of early norepinephrine as an intervention.”
- 55.5% of patients had vasopressors administered through a peripheral IV with no difference in acute limb ischemia or skin necrosis between groups
Author Conclusion: “Early Norepinephrine was significantly associated with increased shock control by 6 hours. Further studies are needed before this approach is introduced in clinical resuscitation practice.”
Clinical Take Home Point: Although, this study confirms my own biases of initiating vasopressor therapy earlier in the course of patients with septic shock, it should be remembered that this study still requires external validation with patient oriented outcomes before implementation into routine clinical practice.
Currently, in my practice, in patients with septic shock, I am starting with a Lactated Ringers bolus and assessing fluid status with RUSH exam. If my patient is euvolemic or hypervolemic, I am beginning my norepinephrine infusion at a much sooner time than waiting for 30cc/kg to be completed
References:
- Permpikul et al. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER): A Randomized Trial. AJRCCM 2019. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- The Bottom Line: CENSER – Early Use of Norepinephrine in septic Shock Resuscitation
- First10EM: CENSER – Early Norepinephrine in Septic Shock
- The SGEM: SGEM #294 – Blood Pressure – Do Better, Keep Rising with Norepi
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)



