 Background: Randomized clinical trials evaluating the efficacy of adjunctive corticosteroids in septic shock have shown conflicting evidence of clinical relevance. Two trials in particular [2][3] looked at lower dose hydrocortisone (200mg/day) and its effect on mortality in patients with septic shock resulting in conflicting results in regards to mortality, but both showing earlier reversal of shock in patients treated with hydrocortisone. The current surviving sepsis guidelines recommend the use of hydrocortisone in patients with septic shock after adequate fluid resuscitation and use of vasopressors who have not achieved hemodynamic stability, but this recommendation is classified as weak evidence (Level 2C). Due to these weak recommendations there has been a variability in use of corticosteroids in septic shock. On Jan 19th, 2018 the ADRENAL Trial results were published trying to once and for all answer the question of adjunctive steroids in septic shock.
Background: Randomized clinical trials evaluating the efficacy of adjunctive corticosteroids in septic shock have shown conflicting evidence of clinical relevance. Two trials in particular [2][3] looked at lower dose hydrocortisone (200mg/day) and its effect on mortality in patients with septic shock resulting in conflicting results in regards to mortality, but both showing earlier reversal of shock in patients treated with hydrocortisone. The current surviving sepsis guidelines recommend the use of hydrocortisone in patients with septic shock after adequate fluid resuscitation and use of vasopressors who have not achieved hemodynamic stability, but this recommendation is classified as weak evidence (Level 2C). Due to these weak recommendations there has been a variability in use of corticosteroids in septic shock. On Jan 19th, 2018 the ADRENAL Trial results were published trying to once and for all answer the question of adjunctive steroids in septic shock.
What They Did:
- International, pragmatic, double-blind, parallel-group randomized, controlled trial of 3800 patients with septic shock undergoing mechanical ventilation
- Hydrocortisone infusion (at a dose of 200mg/day) or placebo for 7 days or until death or discharge from ICU
- ADRENAL = ADjunctive corticosteroid tREatment iN criticAlly ilL patients with septic shock
- Tried to answer the question of whether hydrocortisone therapy reduces mortality in patients admitted to the ICU with septic shock
Outcomes:
- Primary: 90 day mortality
- Secondary:
- 28 day mortality
- Time to resolution of shock
- Recurrence of shock
- Length of ICU stay
- Length of hospital stay
- Frequency and duration of mechanical ventilation
- Frequency and duration of treatment with renal-replacement therapy
- Incidence of new-onset bacteremia or fungemia between 2 and 14 days after randomization
- Blood transfusion requirements
Inclusion:
- Adults (≥18 years of age) undergoing mechanical ventilation with strong clinical suspicion of infection, who fulfilled 2 or more criteria of SIRS
- Continuous vasopressors or inotropes to maintain a SBP >90mmHg or mean MAP >60mmHg, or MAP target set by the treating physician for maintain perfusion
- Administration of vasopressors or inotropes for ≥4 hours and present at time of randomization
Exclusion:
- Receiving systemic corticosteroids for an indication other than septic shock
- Received Etomidate
- Patients receiving treatment with Amphotericin B for systemic fungal infections at time of randomizations
- Patients with documented cerebral malaria
- Patients with documented strongyloides infection
- Considered to suffer death within 90 days from pre-existing disease
- Treatment limitations in place (i.e. DNR)
- Met all inclusion criteria for >24hours
Results:
- 3800 patients from 69 medical-surgical ICUs underwent randomization
- 3658 patients were included in the primary outcome
- 1832 patients in the hydrocortisone group
- 1826 patients in the placebo group
- Admission type breakdown:
- Non-operative (Medical) ≈ 68%
- Operative (Surgical) ≈ 32%
- All patients had similar baseline therapy:
- Mechanical Ventilation ≈ 100%
- Inotropes/Vasopressors ≈ 100%
- Antimicrobials ≈ 98%
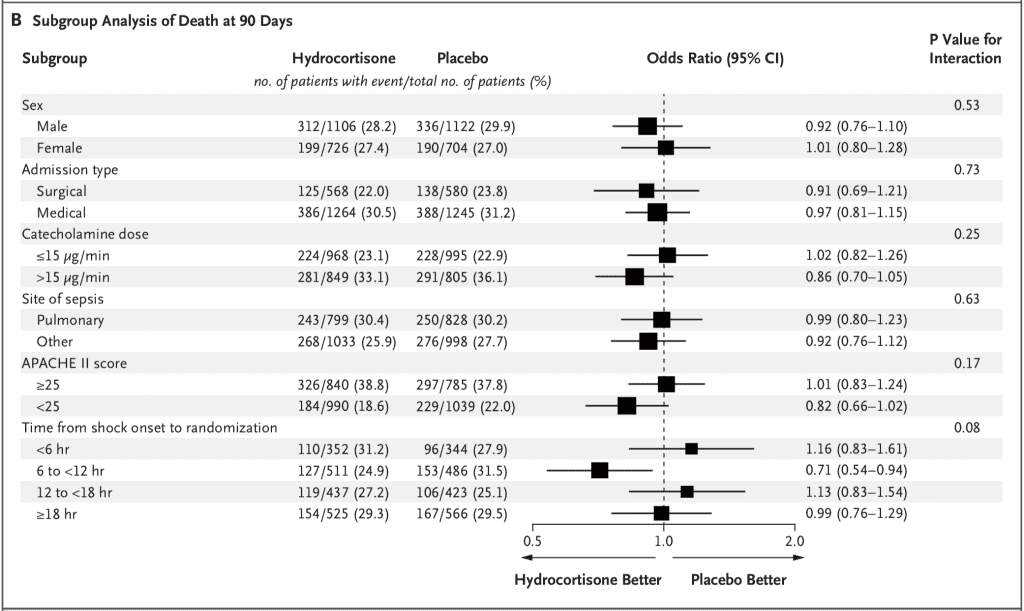
- Death from Any Cause at 90 Days (Primary Outcome):
- Hydrocortisone Group: 27.9%
- Placebo Group: 28.8%
- OR 0.95, 95% CI 0.82 – 1.10; p = 0.50
- 7% of patients received open label steroids, but after doing a post hoc analysis of the primary outcome excluding these patients there is still no difference in mortality OR 0.96, 95% CI 0.82 – 1.12; p = 0.59
- No difference in mortality across 6 pre-specified subgroups as well:
- 3658 patients were included in the primary outcome
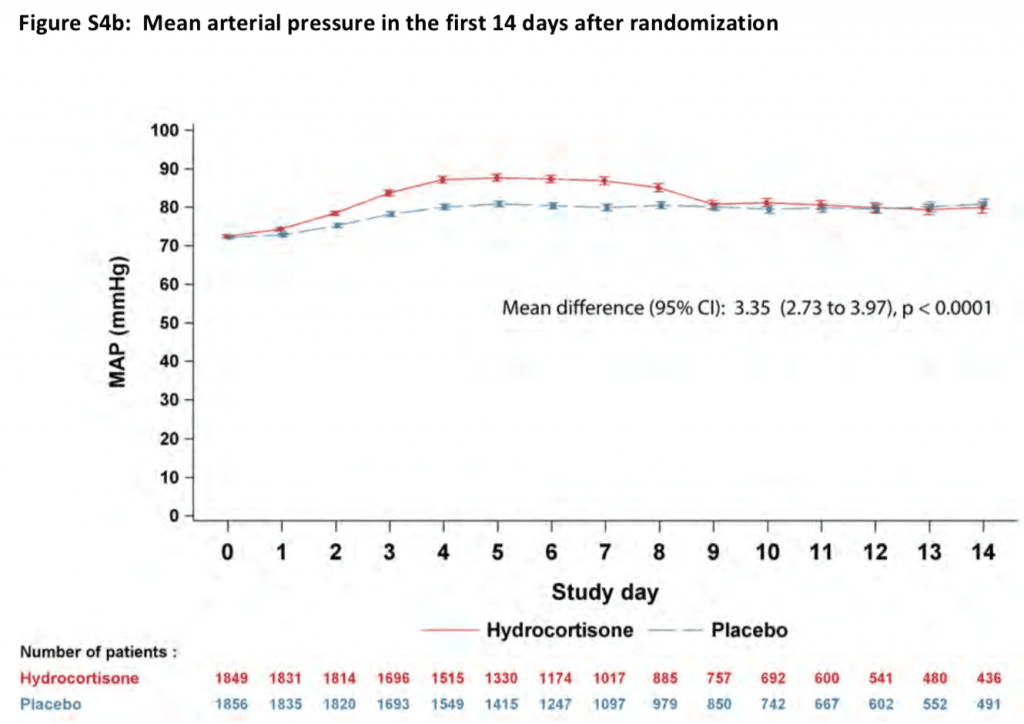
- Median Time to Shock Reversal
- Hydrocortisone Group: 3 Days (Range 2 – 5)
- Placebo Group: 4 Days (Range 2 – 9)
- HR 1.32, 95% CI 1.23 – 1.41; p <0.001
- No Statistical Difference in:
- Mortality at 28 days
- Rate of recurrence of shock
- Number of days alive and out of the ICU
- Number of days alive and out of the hospital
- Recurrence of mechanical ventilation
- Rate of renal replacement therapy
- Incidence of new-onset bacteremia or fungemia
- Adverse Events
- Total of 33 adverse events
- Hydrocortisone Group: 1% (24 events)
- Placebo Group: 0.3% (3 events)
- P = 0.009
- Hyperglycemia
- Hydrocortisone: 6 Events
- Placebo 3 Events
- Serious Adverse Events
- Hydrocortisone Group: 4 Events
- Placebo Group: 2 Events
Strengths:
- International, pragmatic, double-blind, parallel-group randomized, controlled trial increases the validity of the results
- Primary outcome clinically important and patient centered
- Largest patient populations of patients in septic shock to be included in a trial answering the question of adjunctive corticosteroids in septic shock (i.e. 3800 patients)
- Annane Trial [2]: 3.5 years to enroll 299 patients
- CORTICUS Trial [3]: 3.5 years to enroll 499 patients
- VASST Trial: 5 years to enroll 778 patients
- APROCCHS Trial: 6.9 years to enroll 1241 patients
- ADRENAL Trial: 4.1 years to enroll 3800 patients
- Before the trial was even completed the trial protocol and statistical analysis plan was published to help reduce bias in results
- Neither Pfizer (which supplies hydrocortisone) or Radpharm Scientific (which supplied placebo) had any input into the design or conduct of the study, data collection, statistical analysis, or writing the manuscript
- Randomizations concealed using a password-protected, encrypted, web-based interface
- Patients, treating physicians, and trial personnel were blinded in regards to hydrocortisone vs placebo was completed by use of identical, masked vials

- Primary outcome examined in 6 pre-specified subgroups to ensure no subgroup would benefit from hydrocortisone therapy
- Two interim analyses were performed by an independent statistician when 950 patients (25% of enrollment) and 2500 patients (66% of enrollment) could be assessed with regard to the primary outcome
- Baseline characteristics of patients were similar. In other words one patient population was not sicker than the other
- Very few patients lost to follow up (28 patients or 0.7% of study population) increasing the validity of the results
- Results verified in 5 different hospital systems which increases external validity to general practice
Limitations:
- Patients receiving etomidate were excluded from this study. However many providers may still be using etomidate for RSI in septic patients
- Adverse events were recorded based on clinician judgment as being related to the trial regimen, but this judgment was not adjudicated
- Data was not collected on all secondary infections
- The appropriateness of antibiotic therapy was also not adjudicated
- Rates of recurrent ventilation was used as a surrogate for myopathy but long-term neuromuscular weakness was not assessed
- A detailed cost-benefit analysis was not done
- Unclear what amount of fluids patients got in each arm of the study as too much fluid has been associated with increased mortality
- It’s possible that there is a significant difference for a smaller but still clinically relevant outcome (i.e. the 0.9% difference could be real if a larger group was recruited and, across large number of patients may still be important)
- Management outside of steroids at discretion of treating physician, although according to the authors management followed EBM guidelines for sepsis
Discussion:
- This trial was different than many other trials trying to answer this question as they used a hydrocortisone infusion of 200mg/day instead of bolus dosing
- No corticotropin testing was performed due to the controversial nature of this tests results in critically ill patients
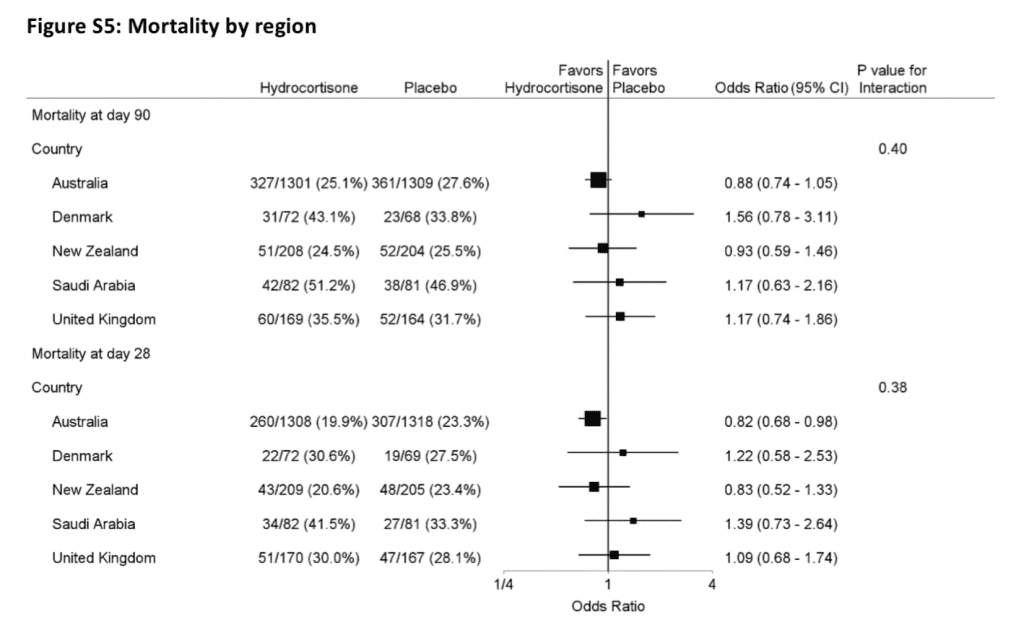
- Breakdown of 90 day mortality by region enrolled (Majority of enrollment came out of Australia ≈ 70%)
- Majority of infection sites were:
- Pulmonary: 33.8% Hydrocortisone group and 36.5% Placebo group
- Abdominal: 25.9% Hydrocortisone group and 25.2% Placebo group
- Some interesting points to consider (And I am not sure we have the answers to these questions):
- Is infusion better than bolus?
- Is the appropriate duration 7 days?
Author Conclusion: “Among patients with septic shock undergoing mechanical ventilation, a continuous infusion of hydrocortisone did not result in lower 90-day mortality than placebo.”
Clinical Take Home Point: In patients with septic shock, admitted to the ICU on mechanical ventilation and requiring ≥4 hours of vasopressors/inotropes, hydrocortisone infusion (200mg/d) compared to placebo, does not make a difference in 90 day mortality, however there were some improved secondary outcomes including: shock reversal, ventilator free days, LOS in the ICU, and fewer blood transfusions required (the last one is hypothesis generating as stated by the authors).
References:
- Venkatesh B et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. NEJM 2018. [Epub Ahead of Print]
- Annane D et al. Effect of Treatment with low Doses of Hydrocortisone and Fludrocortisone on Mortality in Patient with Septic Shock. JAMA 2002. PMID: 12186604
- Sprung CL et al. Hydrocortisone Therapy for Patients with Septic Shock. The CORTICUS Trial. NEJM 2008. PMID: 18184957
For More Thoughts on This Topic Checkout:
- Ryan Radecki at EM Literature of Note: The Definitive Word on Steroids in Septic Shock
- David Slessor at The Bottom Line: Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock
- Rory Spiegel at EMNerd(EMCrit): The Case of the Relative Insufficiency
- Dan Horner at St. Emlyn’s Blog: The End of the ‘Roid?’
- Josh Farkas at PulmCrit (EMCrit): Metabolic Sepsis Resuscitation – Strike Hard, Strike Fast, No Remorse
- Mike Winters at Resuscitation: Episode 89 – Steroids in Septic Shock? Do We Finally Have the Answer?
- Ken Milne at The SGEM: SGEM #208 – It Makes No Difference – Glucocorticoids for the Treatment of Septic Shock
- Rob Macsweeney at Critical Care Reviews (Fast Forward to 1:01):
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



