 Background: Sore throat is a common presentation to the emergency department as well as primary care clinics. Corticosteroids inhibit transcription of pro-inflammatory mediators in airway endothelial cells responsible for pharyngeal inflammation and symptoms of pain. They have been used in other upper respiratory tract infections such as acute sinusitis and croup. In adults, previous studies with dexamethasone are in combination with antibiotics but studies of children have included dexamethasone without antibiotics. This study is unique as it is evaluating the benefits of oral corticosteroids for acute sore throat in primary care in the absence of antibiotics
Background: Sore throat is a common presentation to the emergency department as well as primary care clinics. Corticosteroids inhibit transcription of pro-inflammatory mediators in airway endothelial cells responsible for pharyngeal inflammation and symptoms of pain. They have been used in other upper respiratory tract infections such as acute sinusitis and croup. In adults, previous studies with dexamethasone are in combination with antibiotics but studies of children have included dexamethasone without antibiotics. This study is unique as it is evaluating the benefits of oral corticosteroids for acute sore throat in primary care in the absence of antibiotics
Episode 39 – The TOAST Trial: Dexamethasone for Acute Pharyngitis
Click here for Direct Download of Podcast
Special Guest
 Chip Lange
Chip Lange
Emergency Medicine Physician Assistant (EMPA)
Blog/Podcast: TOTAL EM
Twitter: @the_TOTAL_EM
What They Did:
- Multicenter, double-blind, placebo-controlled, parallel group randomized trial
- Conducted in 42 family practices in England
- Enrolled 576 adult patients with acute sore throat not requiring immediate antibiotic therapy
- Single dose of 10mg of dexamethasone or identical placebo
Outcomes:
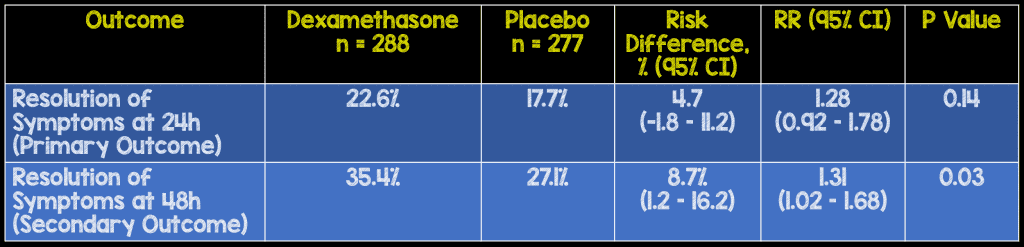
- Primary: Proportion of patients with complete resolution of symptoms at 24hrs
- Secondary:
- Complete resolution at 48hrs
- Duration of moderately bad symptoms (Based on a Likert scale of 0 – 6; 0 = Normal and 6 = bad as it could be)
- Visual Analog Symptom Scales (0 – 100; 0 = No symptoms and 100 = worst imaginable)
- Health care attendance
- Days missed from work or education
- Consumption of delayed antibiotics or other medications
- Adverse events
Inclusion:
- ≥18 years of age
- Presenting to primary care appointment with acute sore throat and odynophagia thought to be infective in origin
- Onset of symptoms within the last 7d
- Capacity and willingness to consent and maintain a symptom diary
Main Exclusions:
- Pregnancy or breast feeding
- <1mo use of inhaled or oral corticosteroids
- <1mo adenotonsillectomy
- <14d taking antibiotics
- Clear alternative diagnosis (i.e. pneumonia)
- Known imunnodeficiency
- Requiring hospital admission
- Requiring immediate antibiotics
- Known dexamethasone allergy
Results:
- 565 adult patients eligible
- Oral Dexamethasone: 288
- Oral Placebo: 277
- Median Age: 34 years (Range: 26 – 45.5 years)
- 75.2% women
- 100% completed the intervention
- No difference in secondary outcomes
Strengths:
- Multicenter, double-blind, placebo-controlled, parallel group randomized trial
- Appropriate sample size to find a 18% increase in resolution with 90% power
- Participants who had no information for the primary outcome were included in the analysis as having no resolution of symptoms
- Primary outcome measure was collected from 93.6% of patients
Limitations:
- A less severely ill patient population may be what was studied due to exclusion of patients requiring immediate antibiotics
- Children were not studied in this trial
- Primary outcome is not a well validated outcome
- Study underpowered to detect a modest effect (<18%) on the primary outcome
Discussion:
- Although there was no difference in the primary outcome of complete resolution of symptoms at 24 hours there was a significant difference in complete resolution of symptoms at 48 hours. When calculating the NNT this gives you a value of 12.
- This study was underpowered for detecting a modest effect on the primary outcome.
- A larger study could potentially identify a statistically significant different between treatments that could not be detected here.
- The study was also not powered to detect a difference in adverse effect profiles.
- Patients in this trial may not have been experiencing severe enough symptoms to receive benefit with dexamethasone.
- Those participating in this study were excluded if they had symptoms or findings that were concerning for infection that required immediate antibiotics.
- This could imply that patients were in general more sick and would have been better patients to benefit from corticosteroids.
- Previous research supports this finding based on how corticosteroids have been used in other studies for adults and children.
- Since this was a study in primary care, patients may not have as severe of symptoms as if they went to the emergency department.
- Patients who present to the emergency department in general may be sicker than those who go to primary care.
- For that potential reason, patients who present to the emergency department may be more likely to benefit just like we specified for those with more severe symptoms in general.
- This could mean that the study is less applicable.
Author Conclusion: “Among adults presenting to primary care with acute sore throat, a single dose of oral dexamethasone compared with placebo did not increase the proportion of patients with resolution of symptoms at 24 hours. However, there was a significant difference at 48 hours.”
Clinical Take Home Point: Corticosteroids may play a beneficial role in sore throat just like in other upper respiratory infections but the effect is more likely to be beneficial in those with more severe symptoms.
References:
- Hayward GN et al. Effect of Oral Dexamethasone Without Immediate Antibiotics vs Placebo on Acute Sore Throat in Adults: A Randomized Clinical Trial. JAMA 2017; 317(15): 1535 – 1543. PMID: 28418482
For More on This Topic Checkout:
- Chip Lange at TOTAL EM: Podcast #53 – Oral Dexamethasone for Sore Throats – A Bonus to a REBEL Cast Post
- Ken Milne at The SGEM: SGEM #203 – Let Me Clear My Sore Throat with a Corticosteroid
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



