 Background: Epinephrine(adrenaline) has been used in advanced life support in cardiac arrest since the early 1960s. Despite the routine recommendation for its use, evidence to support administration is less than ideal. Although it is clear from multiple observational studies that epinephrine improves return of spontaneous circulation (ROSC) and short-term survival, most evidence suggests an absence of improvements in survival with good neurologic outcomes. In cardiac arrest we want to take advantage of the alpha effects of epinephrine, including peripheral vasoconstriction, and therefore increasing aortic diastolic pressure, which in turn helps augment coronary and cerebral blood flow. On the other hand, we want to avoid the potentially detrimental beta effects including dysrhythmias, decreased microcirculation, and increased myocardial oxygen demand all of which increase the chances of recurrent cardiac arrest and decreased neurologic recovery. The only two interventions in cardiac arrest that have shown improve survival with good neurologic outcomes continue to be high-quality CPR and early defibrillation. The debate over the utility of epinephrine in OHCA has been ongoing for several years now and many providers have been awaiting the results of the PARAMEDIC-2 trial that was just published in the NEJM 2018.
Background: Epinephrine(adrenaline) has been used in advanced life support in cardiac arrest since the early 1960s. Despite the routine recommendation for its use, evidence to support administration is less than ideal. Although it is clear from multiple observational studies that epinephrine improves return of spontaneous circulation (ROSC) and short-term survival, most evidence suggests an absence of improvements in survival with good neurologic outcomes. In cardiac arrest we want to take advantage of the alpha effects of epinephrine, including peripheral vasoconstriction, and therefore increasing aortic diastolic pressure, which in turn helps augment coronary and cerebral blood flow. On the other hand, we want to avoid the potentially detrimental beta effects including dysrhythmias, decreased microcirculation, and increased myocardial oxygen demand all of which increase the chances of recurrent cardiac arrest and decreased neurologic recovery. The only two interventions in cardiac arrest that have shown improve survival with good neurologic outcomes continue to be high-quality CPR and early defibrillation. The debate over the utility of epinephrine in OHCA has been ongoing for several years now and many providers have been awaiting the results of the PARAMEDIC-2 trial that was just published in the NEJM 2018.
REBEL Cast Episode 56 – PARAMEDIC-2 – Time to Abandon Epinephrine in OHCA?
Click here for Direct Download of Podcast
What They Did: Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration in Cardiac Arrest (PARAMEDIC-2).This was a multicenter, randomized, double-blind, placebo controlled trial involving 8014 patients with OHCA in the United Kingdom
Patients:Adult patients with OHCA receiving ACLS provided by trial-trained paramedics
Intervention:IV epinephrine 1mg q3 – 5min + standard care
Comparison:IV 0.9% normal saline bolus + standard care
Outcomes:
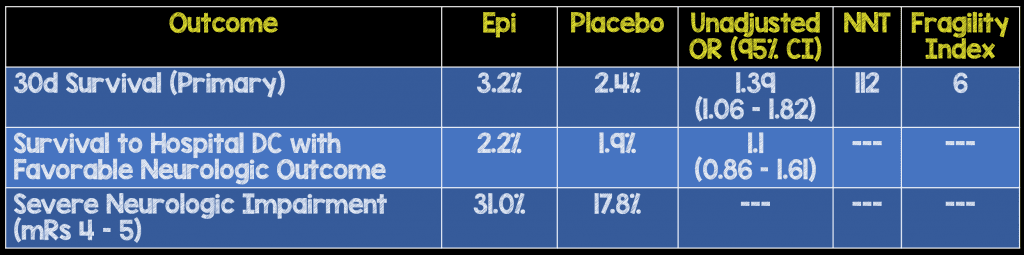
- Primary: 30d Survival
- Secondary:
- Survival to Hospital Discharge with Favorable Neurologic Outcome (i.e. mRS ≤3)
- Survival Until Hospital Admission
- Length of Stay in the Hospital
- Length of Stay in the ICU
- Survival at Hospital Discharge and at 3 Months
- Neurologic Outcomes at Hospital Discharge and at 3 Months
Exclusion:
- Pregnant
- Age <16 years
- Cardiac arrest from anaphylaxis or asthma
- Administration of epinephrine before arrival of a trial-trained paramedic
- Traumatic cardiac arrests excluded in one ambulance service
Results:
- 8014 patients with OHCA
- 4015 in the IV epinephrine arm (Epi)
- 3999 in the IV saline placebo arm (Placebo)
Strengths:
- Asks a clinically important question
- Largest RCT to date on the use of epinephrine in OHCA. Prior to this Jacobs et al trial, which ended prior to complete enrollment was the largest RCT [4]
- Large, multicenter, double-blind, placebo controlled trial
- Data regarding quality of CPR were obtained with use of defibrillator downloads when available
- Outcomes were assessed by research paramedics who were unaware of treatment assignments
- Recorded serious adverse events (death, hospitalization, and disability)
- Interim data and safety monitoring was performed every 3 months
- Patients were well balanced at baseline and had similar concurrent treatments
Limitations:
- Hospital-based care, including targeted temperature management, hemodynamic support, ventilatory criteria, and prognostication were not specified in the trial protocol, but did follow national guidelines
- Used only 1mg epinephrine q3 – 5min, whereas other dosing strategies might have produced different results
- No data on patients baseline neurologic status
- The original protocol anticipated a higher survival rate than the one that was observed (6.5% in the placebo group and 7% in the epinephrine group) àSee discussion for elaboration
- Information about the quality of CPR was limited to the first 5 minutes of cardiac arrest and involved <5% of enrolled patients
Discussion:
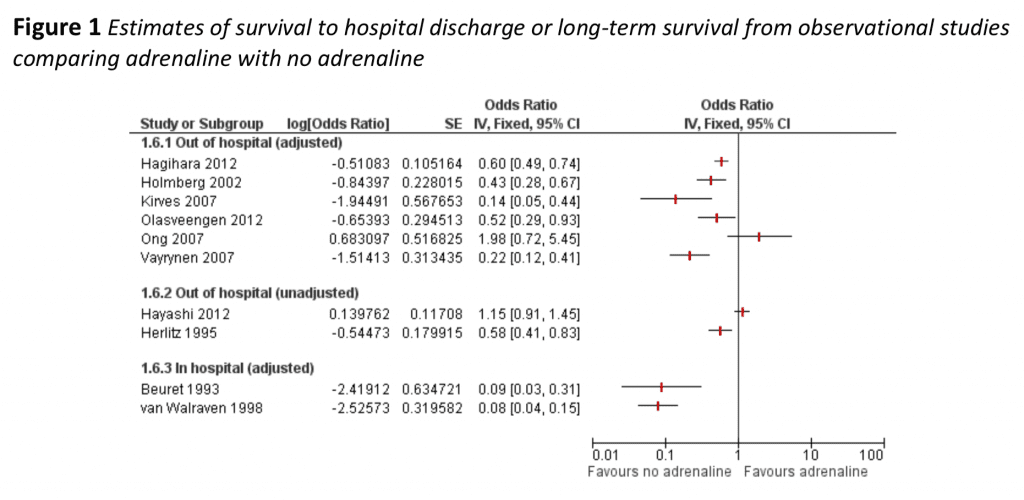
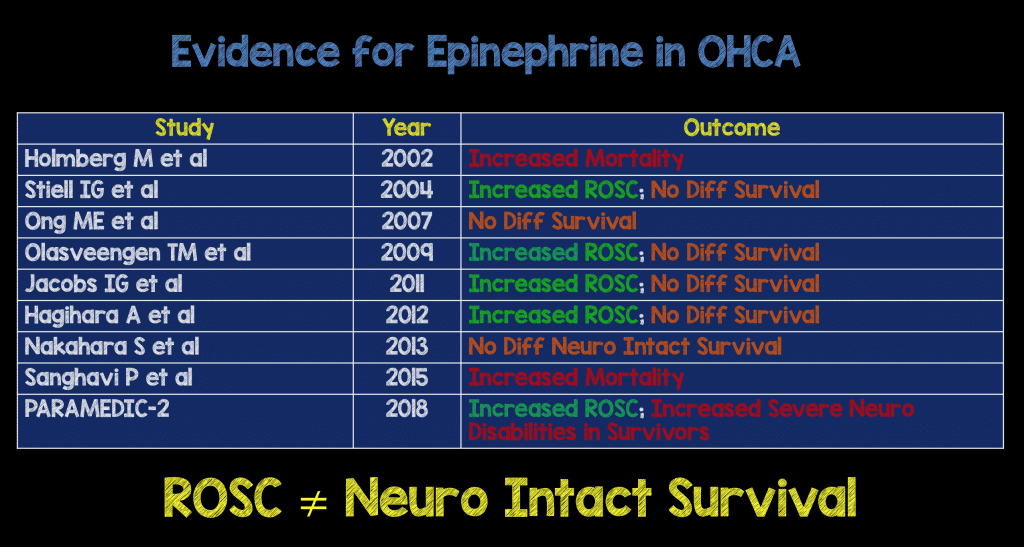
- The evidence to date of survival to hospital discharge or long term survival from prior observational studies:
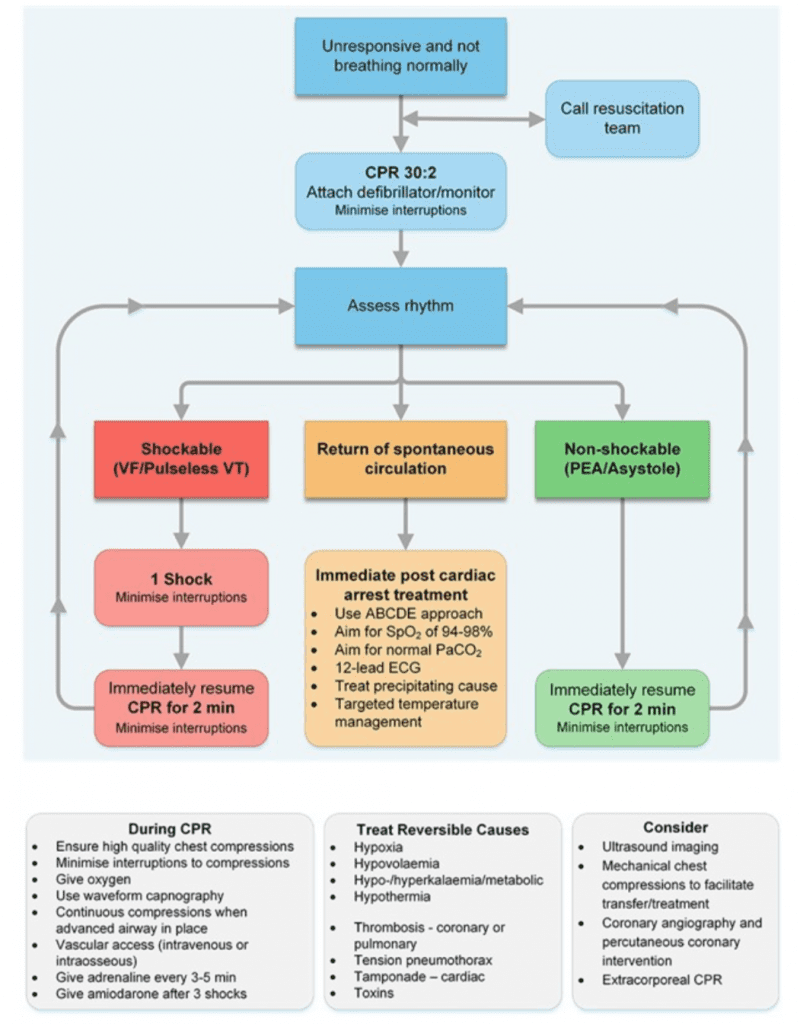
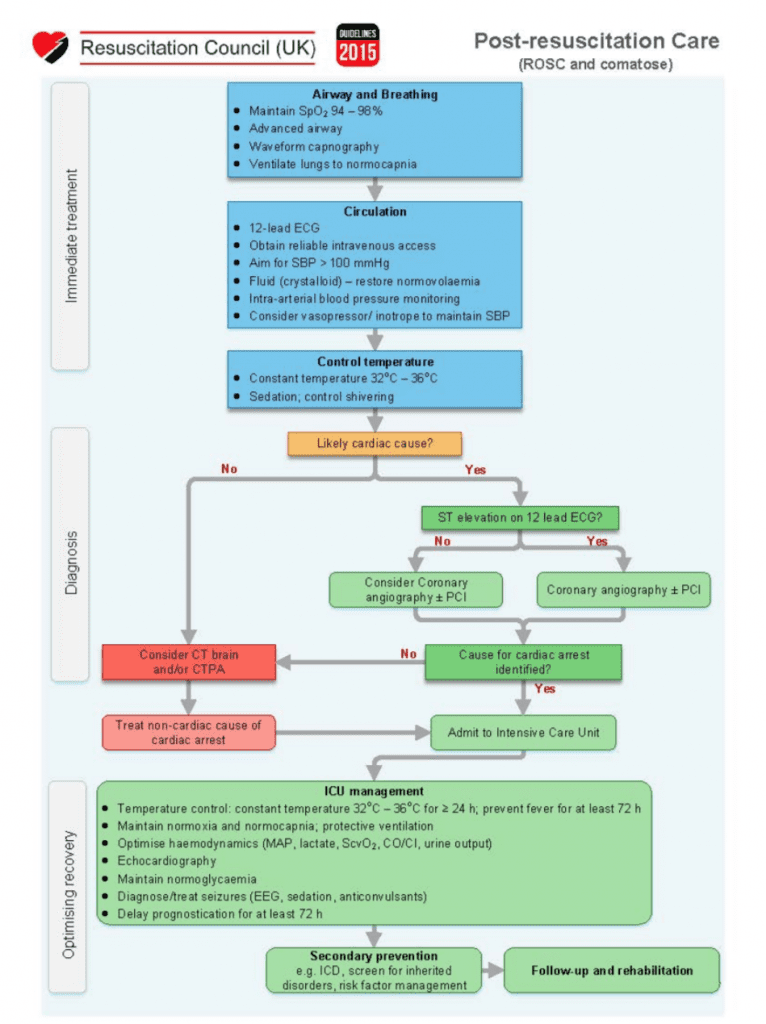
- Algorithms for Resuscitation and Post-ROSC Care:
- There was a low rate of shockable rhythms in this study (19.2% in Epi arm and 18.7% in the placebo arm). This is evidenced by the extremely low survival rate in this study of 3.2% and 2.4% respectively. However, this is interesting because the authors removed 615 patients who survived after initial management (ie defibrillation -> ROSC). This critique seems to be very prevalent on twitter and should be squashed. Survival was low because those most likely to survive, were removed from study.
- CPR was performed in witnessed cardiac arrest in almost 70% of cases
- Median time from emergency call to ambulance arrival at the scene was quite quick at about 6 ½ minutes
- Median time between emergency call and administration of trial agent 21 minutes.This is an important point, as cardiac arrest is believed to have 3 phases: First 5 min = Electrical Phase (Heart Most Amendable to Defib), Next 10 – 15min = Circulatory Phase, and >20min = Metabolic Phase (Acid-Base Derangements). Epinephrine seems to have its best chances of effect at <20min.
- Supraglottic airways were commonly used in this trial in approximately 70% of cases
- As per prior observational trials [2 – 9] the use of epinephrine did seem to increase the chances of ROSC (36.3% vs 11.7%).
- The authors also make the astute point that number of patients who would need to be treated with epinephrine to prevent one death after cardiac arrest was 112 in this study, but this should also be compared to the things that make a difference in neurologic outcomes which include, CPR performed by a bystander (NNT = 15) and early defibrillation (NNT = 5)
Author Conclusion:“In adults with out-of-hospital cardiac arrest, the use of epinephrine resulted in a significantly higher rate of 30-day survival than the use of placebo, but there was no significant between-group difference in the rate of a favorable neurologic outcome because more survivors had severe neurologic impairment in the epinephrine group.”
Clinical Take Home Point: In this well done, multicenter trial epinephrine given at 1mg q3 – 5 minutes increased the chances of ROSC but came at a cost of more ICU usage, more patients with severe neurological disabilities, and no difference in survival with favorable neurological outcome compared to placebo. The use of epinephrine 1mg q 3 – 5 minutes should no longer be part of standard cardiac arrest protocols. Epinephrine should be administered on a case by case basis by experienced providers who perceive that there is a benefit to be had.
References:
- Perkins GD et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. NEJM 2018. [Epub Ahead of Print]
- Hagihara A et al. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 2012. PMID: 22436956
- Holmberg M et al. Low chance of survival among patients requiring adrenaline (epinephrine) or intubation after out-of-hospital cardiac arrest in Sweden.Resuscitation2002. PMID: 12104107.
- Jacobs I et al. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial.Resuscitation 2011. PMID: 21745533.
- Olasveengen T et al. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial.JAMA 2009. PMID: 19934423
- Ong M et al. Survival outcomes with the introduction of intravenous epinephrine in the management of out-of-hospital cardiac arrest.Annals of emergency medicine 2007. PMID: 17509730
- Stiell IG et al. Advanced Cardiac Life Support in Out-of-Hospital Cardiac Arrest in OHCA. NEJM 2004. PMID: 15306666
- Nakahara S et al. Evaluation of Pre-Hospital Administration of Adrenaline (Epinephrine) by Emergency medical Services for Patients with Out-of-Hospital Cardiac Arrest in Japan: Controlled Propensity Matched Retrospective Cohort Study. BMJ 2013. PMID: 24326886
- Sanghavi P et al. Outcomes after Out-of-Hospital Cardiac Arrest Treated by Basic vs Advanced Life Support. JAMA Intern Med 2015. PMID: 25419698
For More Thoughts on This Topic Checkout:
- Simon Carley at St. Emlyn’s Blog: JC – Does Epinephrine Work in Cardiac Arrest
- Justin Morgenstern at First10EM: PARAMEDIC-2 – Epinephrine Harms/Helps in OOHCA
- Rory Spiegel at EMNerd (EMCrit): The Case oft he Costly Compound
- The Resus Room: PARAMEDIC2
- Chip Lange at TOTAL EM: PARAMEDIC2 – It’s Time to Call the Code on Epinephrine (Adrenaline)
- George Walker at The Bottom Line: PARAMEDIC2 – Adrenaline vs Placebo
- Ken Milne at The SGEM: SGEM #238 – The Episode Don’t Work for OHCA
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)




