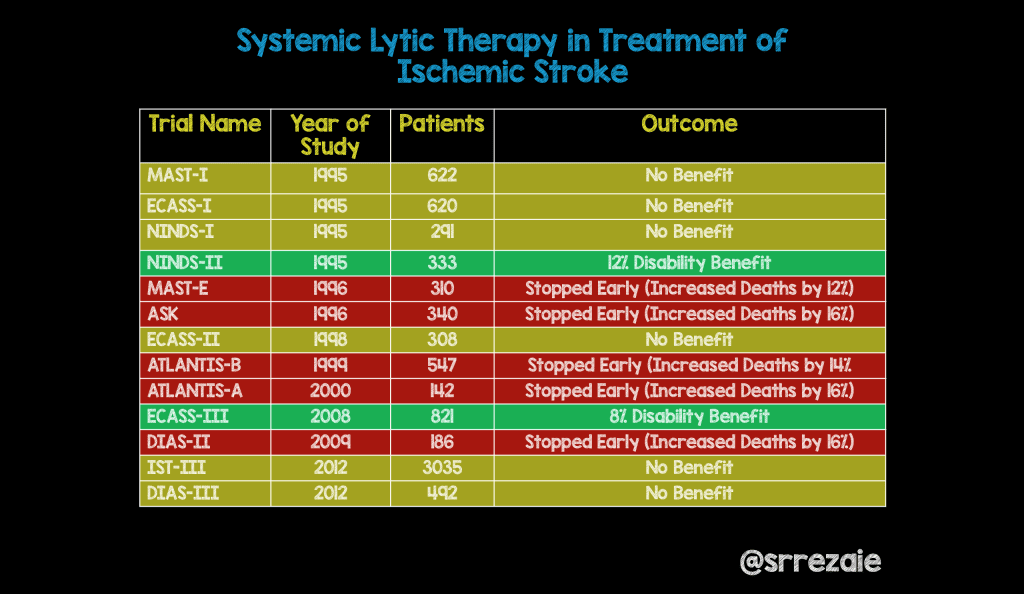
Systemic Lytic Therapy in the Treatment of Ischemic Stroke
What does the American College of Emergency Physicians (ACEP) 2015 Clinical Policy Say? [PDF HERE]
- Is IV tPA safe and effective for patients with acute ischemic stroke if given within 3 hours of symptom onset?
- Level A recommendations: None specified.
- Level B recommendations: With a goal to improve functional outcomes, IV tPA should be offered and may be given to selected patients with acute ischemic stroke within 3 hours after symptom onset at institutions where systems are in place to safely administer the medication. The increased risk of symptomatic intracerebral hemorrhage (sICH) should be considered when deciding whether to administer IV tPA to patients with acute ischemic stroke.
- Level C recommendations: When feasible, shared decision making between the patient (and/or his or her surrogate) and a member of the health care team should include a discussion of potential benefits and harms prior to the decision whether to administer IV tPA for acute ischemic stroke (Consensus recommendation).
- Is IV tPA safe and effective for patients with acute ischemic stroke treated between 3 to 4.5 hours after symptom onset?
- Level A recommendations: None specified.
- Level B recommendations: Despite the known risk of symptomatic intracerebral hemorrhage (sICH) and the variability in the degree of benefit in functional outcomes, IV tPA may be offered and may be given to carefully selected patients with acute ischemic stroke within 3 to 4.5 hours after symptom onset at institutions where systems are in place to safely administer the medication.
- Level C recommendations: When feasible, shared decision making between the patient (and/or his or her surrogate) and a member of the health care team should include a discussion of potential benefits and harms prior to the decision whether to administer IV tPA for acute ischemic stroke (Consensus recommendation).
Endovascular Treatment of Ischemic Stroke
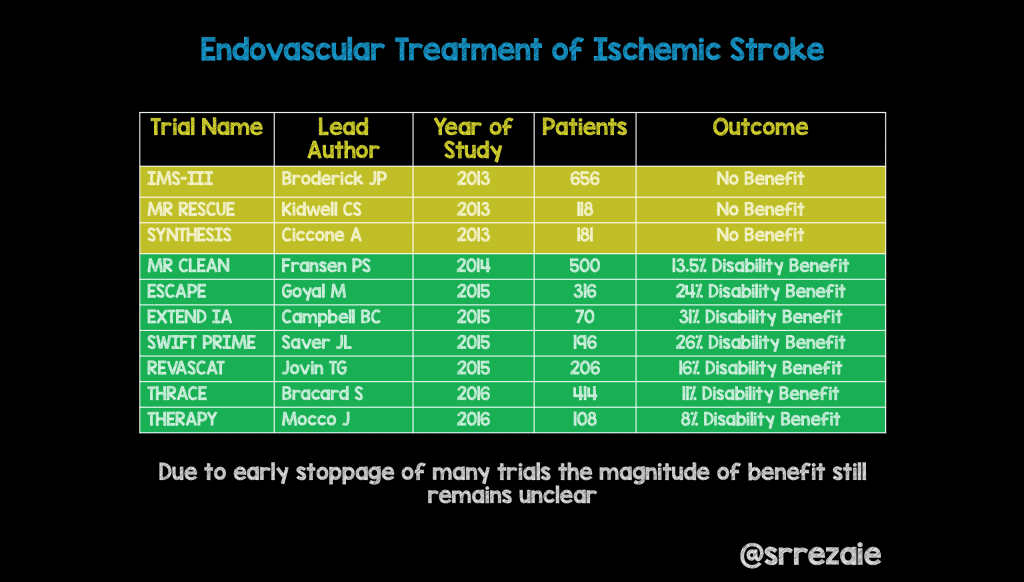
What should we take away from the above endovascular studies?
- Only a very limited population will benefit from endovascular therapy
- Patients with “Severe Strokes”
- Proximal Large Vessel Occlusions (Anterior Circulation)
- Salvageable Brain Tissue (Small Infarcted Core and Collateral Blood Flow)
For More on These Topics Checkout:
Systemic tPA in the Treatment of Ischemic Stroke
- Ken Milne and Anand Swaminathan at The SGEM: SGEM#70 – The Secret of NINDS (Thrombolysis for Acute Stroke)
- Scott Weingart at EMCrit: Podcast 116 – the tPA for Ischemic Stroke Debate
- Jeremy Faust and Lauren Westafer at FOAMCast: FOAMCastini – ACEP TPA Clinical Policy
- Jeremy Faust and Lauren Westafer at FOAMCast: FOAMCastini – Dr. Jerome Hoffman on ACEP’s TPA Clinical Policy
- David Newman at SMART EM: SMART Thrombolytics for Acute Stroke
- Rory Spiegel at EM NERD: The Case of the Non-Inferior Inferiority Continues
- Josh Farkas at PulmCrit/EMCrit: What is the Fragility Index of the NINDS Trial?
- Kyle Dewitt at Emergency Medicine Pharmd: Mirror Mirror on the Wall, who’s the Most Fragile of them All? Assessing the Fragility Index of ECASS III
- Ali G at St. Emlyn’s Blog: JC – Kicking Back on Stroke Thrombolysis
- Justin Morgenstern at First 10 EM: Thrombolytics for Stroke – The Evidence
- Anton Helman At Emergency Medicine Cases: Journal Jam 10 – Thrombolysis & Endovascular Therapy for Stroke Part 1
- Anton Helman at Emergency Medicine Cases: Journal Jam 10 – Thrombolysis & endovascular Therapy for Stroke Part 2
Endovascular Treatment of Ischemic Stroke
- Wesley George at EMDocs: Endovascular Stroke Therapy – Is This The New Standard?
- Ryan Radecki at EMLit of Note: It’s Stroke Week Again!
- Ryan Radecki at EMLit of Note: Christmas Comes Early for Endovascular Therapy in Stroke
- Rory Spiegel at EM Nerd: The Adventure of the Cardboard Box Revisited
- Ken Milne at The SGEM: SGEM#137 – A Foggy Day – Endovascular Treatment for Acute Ischemic Stroke
- Rory Spiegel at EMCrit/EM Nerd: The Case of the Erratic Pendulum
- Justin Morgenstern at First 10 EM: Interventional Therapy for Acute Ischemic Stroke – The Evidence
Disclaimer: This is by no means every piece of literature on the treatment of ischemic stroke, but instead a start to an archive that can continuously be updated. I was hoping the FOAM community might be willing to help out, to make this an even more robust evidence based archive that we can reference for future needs. Feel free to leave comments below, all help is welcome, and if there is literature not included feel free to put links in the comments as well. Appreciate everyone’s help and viva la FOAM!!!!
References:
- Multicentre Acute Stroke Trial – Italy (MAST-I) Group. Randomized Controlled Trial of Streptokinase, Aspirin, and Combination of Both in Treatment of Acute ISchaemic Stroke. Multicentre Acute Stroke Trial-Italy (MAST-I) Group. Lancet 1995; 346(8989): 1509 – 14. PMID: 7491044
- Hacke W et al. Intravenous Thrombolysis with Recombinant Tissue Plasminogen Activator for Acute Hemispheric Stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274(13): 1017 – 25. PMID: 7563451
- The National Institute of Neurological Disorders and stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. NEJM 1995; 333(24): 1581 – 7. PMID: 7477192
- Multicentre Acute Stroke Trial – Europe (MAST-E) Group. Thrombolytic Therapy with Streptokinase in Acute Ischemic Stroke. NEJM 1996; 335(3): 145 – 50. PMID: 8657211
- Donnan GA et al. Streptokinase for Acute Ischmeic Stroke with Relationship to Time of Administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996; 276(12): 961 – 6. PMID: 8805730
- Hacke W et al. Randomised Double-Biind Placebo-Controlled Trial of Thrombolytic Therapy with Intravenous Alteplase in Acute Ischemic Stroke (ECASSII). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352(9136): 1245 – 51. PMID: 9788453
- Clark WM et al. Recombinant Tissue-Type Plasminogen Activator (Alteplase) for Ischemic Stroke 3 to 5 Hours After Symptom Onset. The ATLANTIS STudy: A Randomized Controlled Trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999; 282(21): 2019 – 26. PMID: 10591384
- Clark WM et al. The rtPA (Alteplase) 0- to 6-Hour Acute STroke Trial, Part A (A0276g): Results of a Double-Blind, Placebo-Controlled, Multicenter Study. Thromblytic Therapy in Acute Ischemic Stroke Study Investigators. Stroke 2000; 31(4): 811 – 6. PMID: 10753980
- Hacke W et al. Thrombolysis with Alteplase 3 to 4.5 Hours After Ischemic stroke. NEJM 2008; 359(13): 1317 – 29. PMID: 18815396
- Hacke W et al. Intravenous Desmoteplase in Patients with Acute ISchaemic Stroke Selected by MRI Perfusion-Diffusion Weighted Imaging or Perfusion CT (DIAS-2): A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Lancet Neurol 2009; 8(2): 141 – 50. PMID: 19097942
- IST-3 Collaborative Group. The Benefits and Harms of Intravenous Thrombolysis with Recombinant Tissue Plasminogen Activator Within 6 h of Acute ISchaemic Stroke (The Third International Stroke Trial [IST-3]): A Randomized Controlled Trial. Lancet 2012; 379(9834): 2352 – 63. PMID: 22632908
- Albers GW et al. Safety and Efficacy of Desmoteplase Given 3-9 h After Ischemic Stroke in Patients with Occlusion or High-Grade Stenosis in Major Cereral Arteries (DIAS-3): A Double-Blind, Randomized, Placebo-Controlled Phase 3 Trial. Lancet Neurol 2015; 14(6): 575 – 84. PMID: 25937443
- Broderick JP et al. Endovascular Therapy After Intravenous t-PA Versus t-PA Alone for Stroke. NEJM 2013; 368(10): 893 – 903. PMID: 23390923
- Kidwell CS et al. A Trial of Imaging Selection and Endovascular Treatment for Ischemic Stroke. NEJM 2013; 368(10): 914 – 23. PMID: 23394476
- Ciccone A et al. Endovascular Treatment for Acute Ischemic Stroke. NEJM 2013; 368(10): 904 – 13. PMID: 23387822
- Fransen PS et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. NEJM 2015; 372(1): 11 – 20. PMID: 25517348
- Goyal M et al. Randomized Assessment of rapid Endovascular Treatment of Ischemic Stroke. NEJM 2015; 372(11): 1019 – 30. PMID: 25671798
- Campbell BC et al. Endovascular Therapy for ISchemic Stroke with Perfusion-Imaging Selection. NEJM 2015; 372(11): 1009 – 18. PMID: 25671797
- Saver JL et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs t-PA Alone in Stroke. NEJM 2015; 372(24): 2285 – 95. PMID: 25882376
- Jovin TG et al. Thrombectomy Within 8 Hours After Symptom Onset in Ischemic Stroke. NEJM 2015; 372(24): 2296 – 306. PMID: 25882510
- Badhiwala JH et al. Endovascular Thrombectomy for Ischemic Stroke: A Meta-Analysis. JAMA 2015; 314(17): 1832 – 43. [epub ahead of print]
- Anderson CS et al. Low-Dose vs Stnadard-Dose Intravenous Alteplase in Acute Ischemic Stroke. NEJM 2016; [epub ahead of print] PMID: 2761018
- Bracard S et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurology. [Epub ahead of print]
- mocco J et al. Aspiration Thrombectomy After Intravenous Alteplase Versus Intravenous Alteplase Alone. Stroke. 2016;47(9):2331-8. PMID: 27486173
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



