The Impella Pump Setup
- What is it?: An intravascular microaxial blood pump that provides temporary mechanical circulatory support to reduce workload of the heart while improving systemic circulation
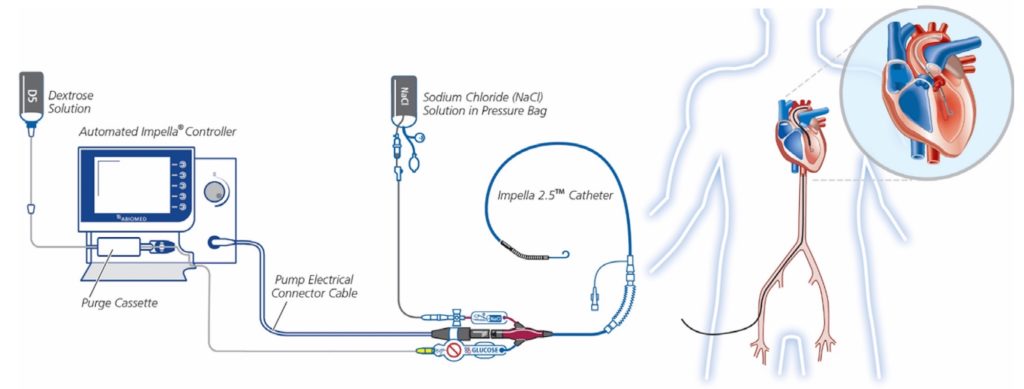
Image from [5]
The Impella Pump/Physiology
- Technology is load dependent, not rhythm dependent (The rhythm aspect distinguishes these devices from IABPs)
- Afterload sensitive (i.e. flow through the pump decreases with increasing ventriculo-aortic pressure gradient)
- This accounts for phasic motor current fluctuations during the cardiac cycle
- Highest pump flow and motor current achieved during systole (when gradient between LV and aorta is minimal)
- Preload dependent (pump needs sufficient inflow for normal pump output)
- Left Sided Device
- Positioned across the aortic valve into the left ventricle
- Provides continuous anterograde blood flow from the LV into the ascending aorta
- Unloads left ventricle = Reduces LVEDP and LV wall tension which reduces LV work and myocardial oxygen demand
- Increases MAP, diastolic pressure, and cardiac output (CO) which increases cardiac power output leading to improved systemic perfusion and increased coronary flow
- Decrease in pulmonary capillary pressure which reduces RV afterload
- Right Sided Device
- Delivers blood from the inlet of the inferior vena cava into the pulmonary artery
- Impella Device Position
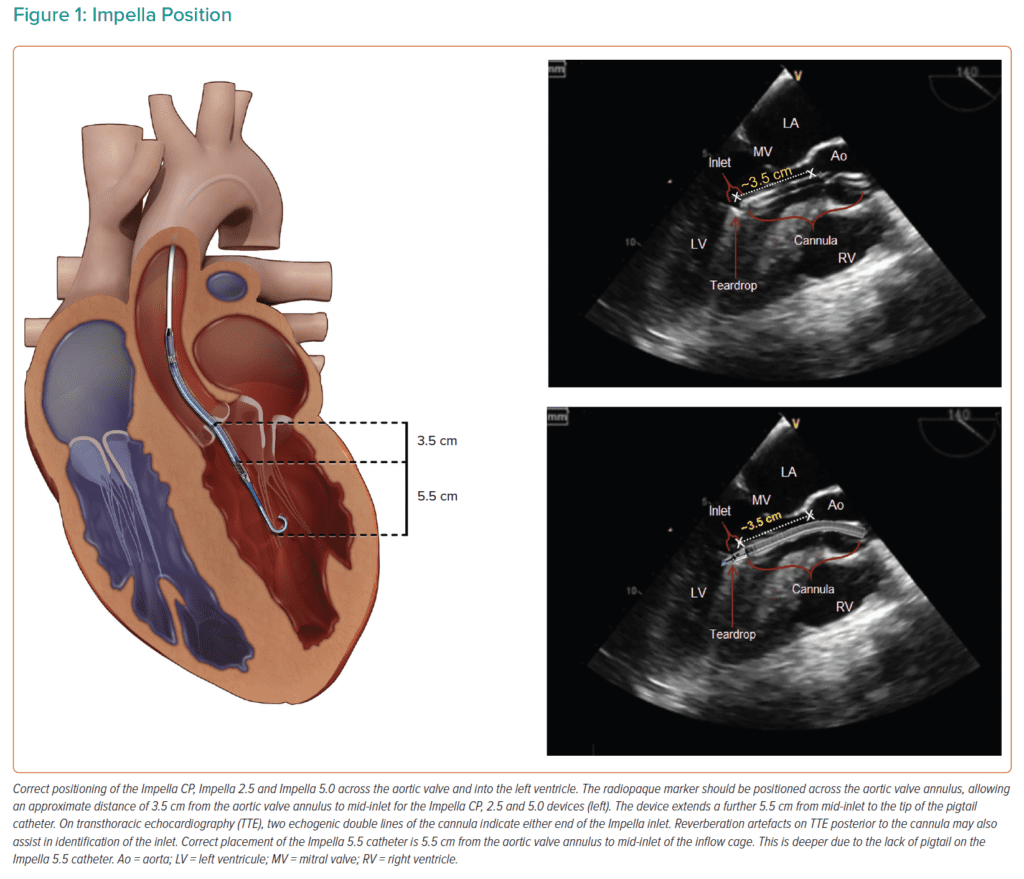
Image from [2]
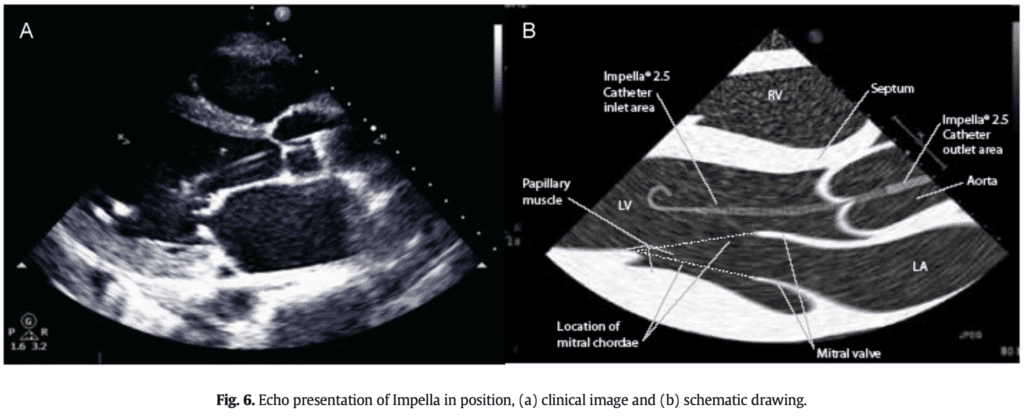
Image from [4]
Types of Impella Devices
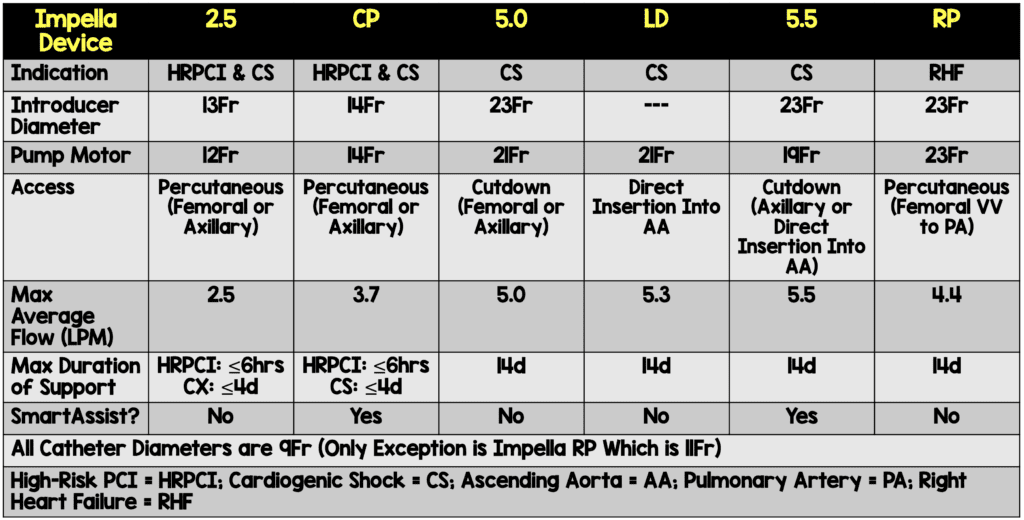
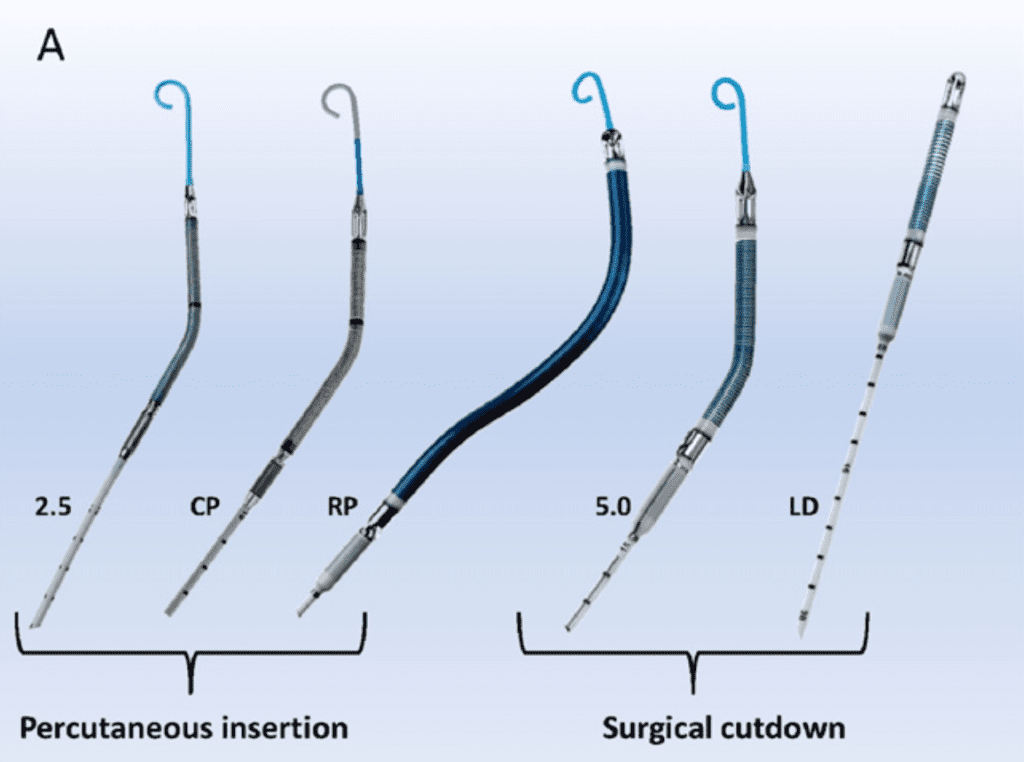
Image from [4]
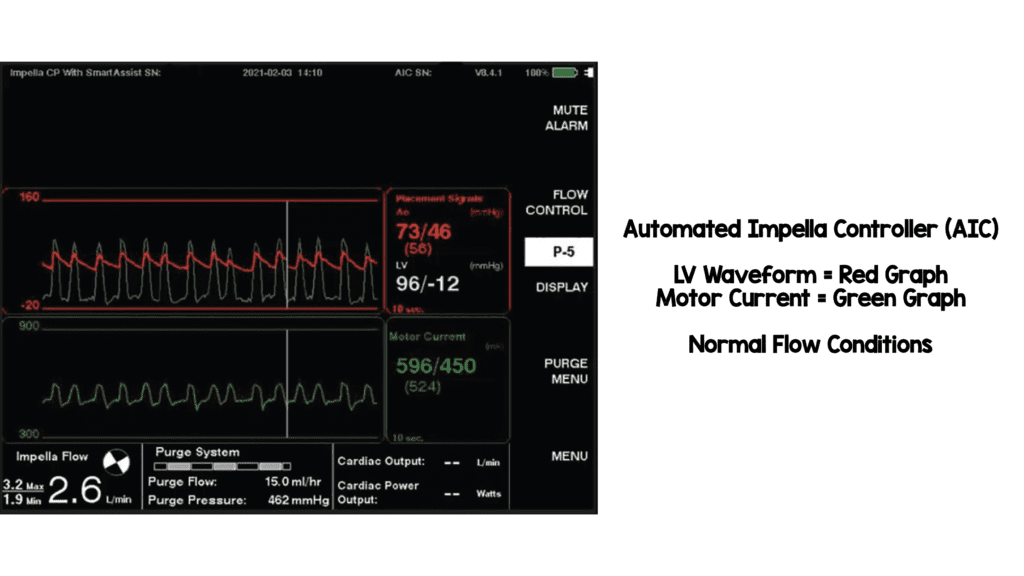
- Automated Impella Controller with SmartAssist Technology
- Automated Impella Controller = Primary user control interface for all Impella heart pumps
- Impella Connect = Cloud-based platform that allows for secure remote viewing and collaborative patient management
- Impella CP and 5.5 have SmartAssist technology (real-time display of informative pump metrics and device placement on the Automated Impella Controller)
- Left-sided Impella devices with SmartAssist technology sense the pressure at the outlet of the device (i.e. aortic pressure when in the correct position) and provide exact device positioning
- The microaxial motor can also sense the pressure difference between the inlet and outlet of the impella device (When in the proper position, this is the pressure difference between the aorta and left ventricle)
- Right-sided impella devices with SmartAssist technology
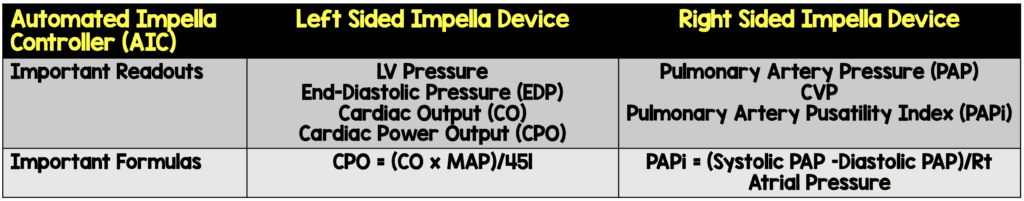
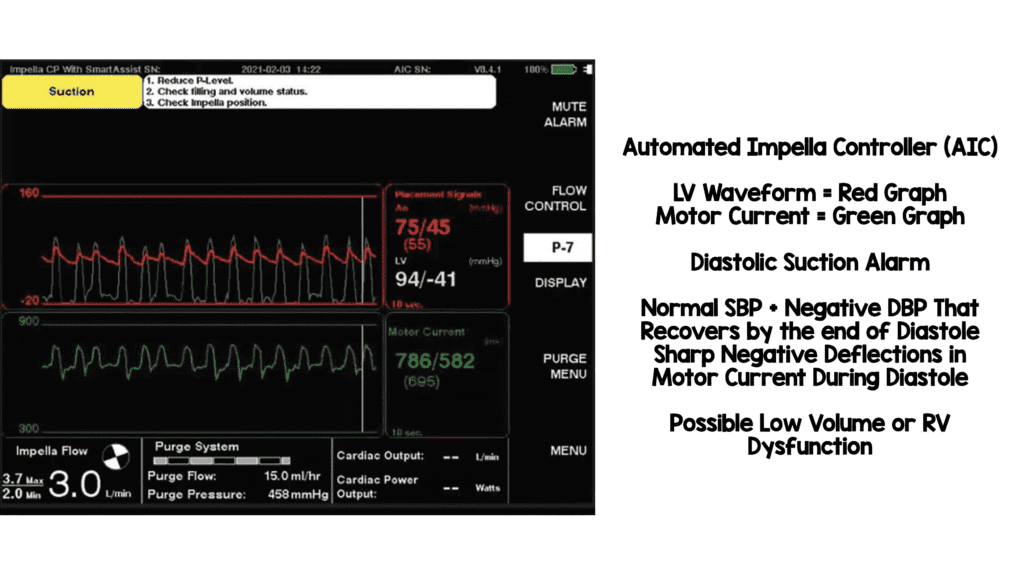
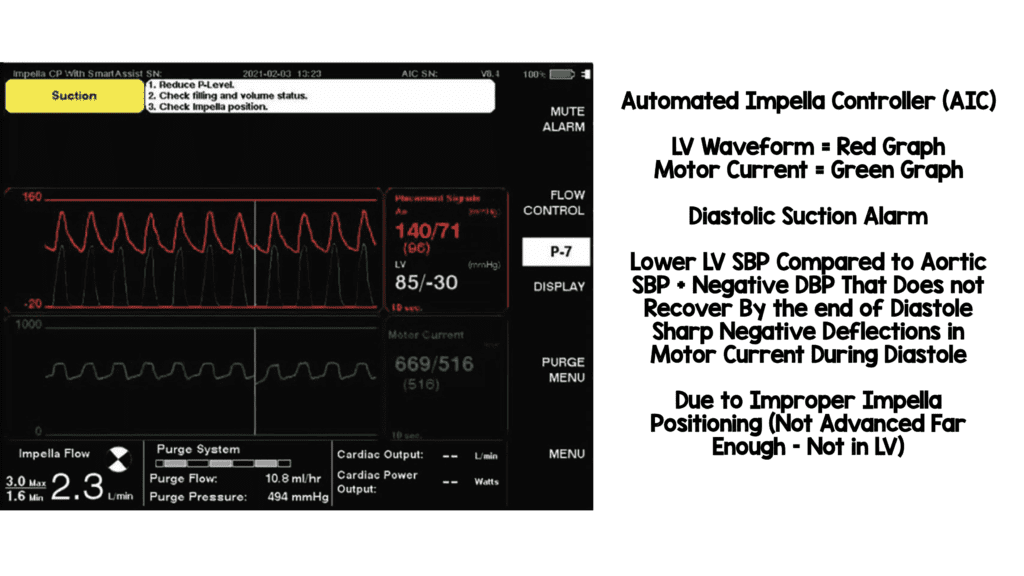
Images Modified from [2]
Indications:
- High-risk PCI (HRPCI):
- Patients deemed ineligible for surgery or long procedural times (i.e. atherectomy or extensive stenting)
- PROTECT II [1]: 1st RCT comparing outcomes with different forms of mechanical circulatory support undergoing high-risk PCI
- No significant difference in primary outcome (30d major adverse event rate)
- BUT patients supported with the Impella 2.5 showed better performance on a prespecified analysis of the primary endpoint at 90d
- Cardiogenic Shock:
- Patients demonstrate better hemodynamic parameters (i.e. CI, CO, and MAP)
- No consensus on optimal time to initiate mechanical circulatory support for patients with cardiogenic shock undergoing PCI
- Pulmonary artery catheterization recommended for use with mechanical circulatory support in cardiogenic shock to help monitor effectiveness, optimize device settings, determine escalation requirements, and assist decision making on weaning
- RV Failure:
- Impella RP with pulmonary artery catheter
- Not easy to identify patients, but one invasive hemodynamic parameter is PAPi
- The most commonly used cut-off value of PAPi is ≤0.9 [2]
- Sensitivity 100% and Specificity 98% for predicting in-hospital mortality and/or requirement for RV support
Benefits:
- Can produce higher flow than IABP
Contraindications:
- Severe Aortic stenosis(Valve Area ≤0.6cm2)
- Although more commonly used in conjunction with TAVR
- Severe Aortic regurgitation (Need competent valve to enable optimal Impella mediated flow forward)
- Severe AR can result in increased aortic pressure which may increase AR and worsen aortic and LV dilation
- Mechanical Aortic valve
- LV thrombus (Device could potentially ingest clot resulting in the device not working/failing)
Impella Implantation Techniques:
- Femoral Access
- Most common approach
- Impella 2.5 and Impella CP inserted using 13F and 14F sheaths respectively via a retrograde femoral artery approach
- Catheter is inserted percutaneously through the femoral artery into the ascending aorta, across the aortic valve, and into the LV (guided by fluoroscopic or echocardiographic imaging)
- Impella 5.0 uses a 23F sheath but more commonly inserted via axillary access
- Axillary Access
- Used when femoral access is precluded due to peripheral arterial disease
- Not as restrictive to ambulation as femoral artery insertion
- The larger sheaths of Impella 5.0 and Impella 5.5 require surgical cutdown
- Surgical and Transcaval Implantation
- Impella 5.0, 5.5, and LD use a 23F sheath and require surgical cutdown arteriotomy or a transcaval approach
- Impella LD is exclusively surgically inserted into the ascending aorta through a 10mm vascular graft and advanced across the valve into the LV
- Right Heart Access
- Impella RP is a 23F pump on an 11F catheter that is inserted percutaneously through the femoral vein into the pulmonary artery guided by fluoroscopic imaging
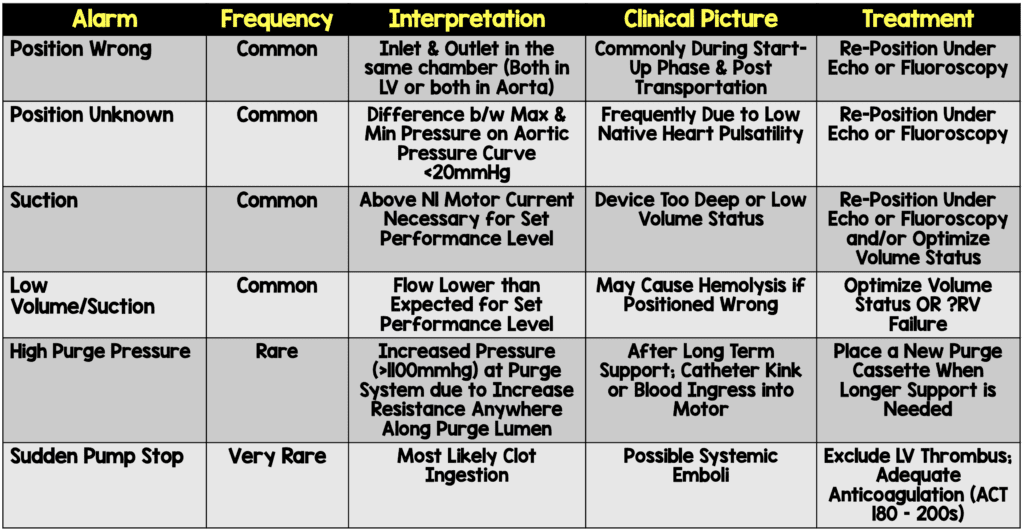
- LV Device Repositioning
- Transthoracic echocardiography should be used to visualize the device
- SmartAssist technology on the Impella CP or Impella 5.5 devices can help with this as well
- Retract the device until diastolic pressures normalizes
- If TTE imaging is difficult, fluoroscopy or TEE can also be used
- Single Access Technique
- PCI catheter up to 7F is inserted through the valve of the 14F Impella CP sheath parallel to the catheter
Device Weaning and Escalation:
- There are no universally accepted guidelines for weaning
- Cardiac Power Output (CPO) has been shown to be the strongest predictor of mortality in patients receiving mechanical cardiac support for cardiogenic shock [2]
- One weaning/escalation protocol used at Ascension St. John Hospital and Medical Center

Modified from [2]
Anticoagulation Strategies:
- Systemic anticoagulation + Anticoagulant added to the purge fluid
- Some patients, require the addition of titratable, supplemental IV heparin to provide optimal anticoagulation
Stonybrook Anticoagulation with Heparin Protocol for Impella

Image from [7]
- Purge solution with added anticoagulant prevents blood entrance into the motor chamber and maintains the patency of the purge solution channel
- Current recommendation for the initial purge solution is heparin 25U/mL in 5% dextrose [2]
How the Purge System Works (4min video)
- Patients with heparin-induced thrombocytopenia (HIT) = Bivalirudin or Argatroban
- Bivalirudin
- Half-Life = 25min (with normal renal function)
- Mechanism of Action = Direct Thrombin Inhibition
- Protein Binding = None
- Metabolism/Excretion: Combo of proteolytic cleavage + Renal
- Monitor with PTT level
- No Antidote BUT short half live (Back to baseline coagulation at ≈1hr)
- No risk of HIT
- Bivalirudin
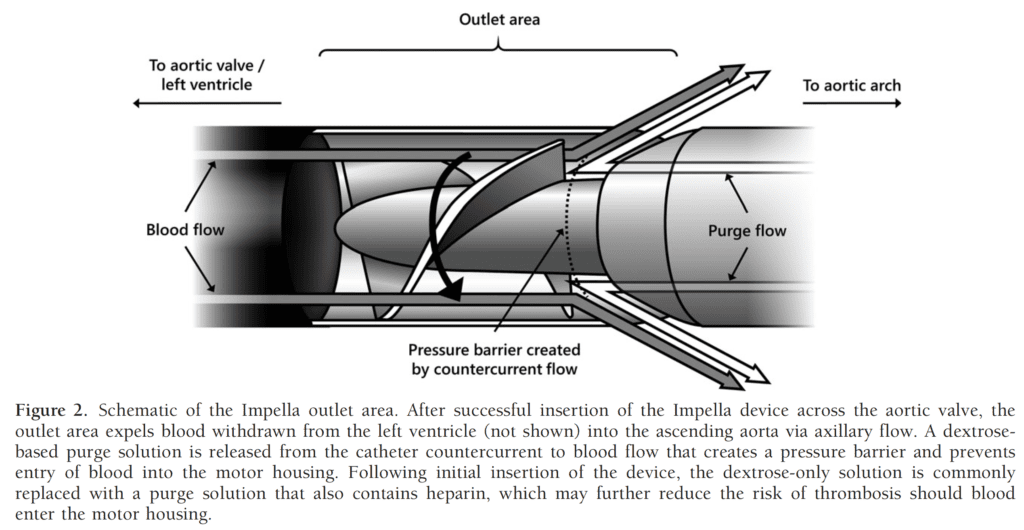
Image from [5]
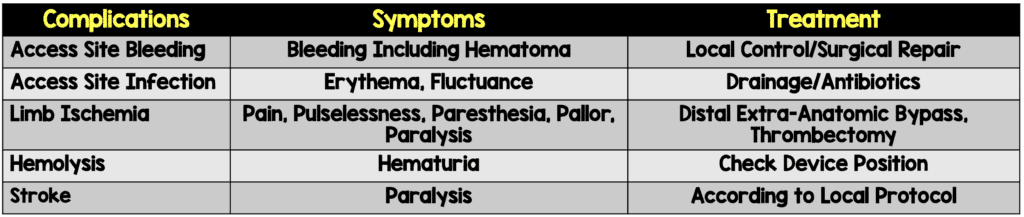
Complications:
- Pump migration
- Ventricular Arrhythmia
- Limb Ischemia
- Pericardial tamponade
- Infection/Sepsis
- Hematoma formation/vascular injury
- May require surgery to repair
- Bleeding [2]
- Median rate of major bleeding complications = 5.2%
- Median rate of major vascular complications = 2.6%
- Thrombosis
- Mechanical hemolysis
- Check plasma free hemoglobin, LDH, haptoglobin, and bilirubin
- Treatment: Confirm device positioning, administration of IV fluids, reduction in level of Impella support, titration of inotropes
- Thrombocytopenia (Due to heparin)
- Consider transition to Bivalirudin
Checklist Upon Arrival to ICU:
- Make sure Tuohy-Borst valve on Impella catheter is locked
- Perform TTE to ensure optimal impella position
- Disable Autoflow feature on console and switch to P-level mode with as high support as possible (P8)
- Disable suction control
- Transfer the purge system to “standard configuration”
- Ensure the roller clap on pressure bag is open
- Verify pressure bag pressure is 300 to 350mmHg
- Make sure the Impella catheter is secured
References:
- O’Neill WW et al. A Prospective, Randomized Clinical Trial of Hemodynamic Support with Impella 2.5 Versus Intra-Aortic Balloon Pump in Patients Undergoing High-Risk Percutaneous Coronary Intervention: The PROTECT II Study. Circulation 2012. PMID: 22935569
- Zein R et al. A Review of the Impella Devices. Interv Cardiol 2022. PMID: 35474971
- Baran DA et al. Temporary Mechanical Circulatory Support: Devices, Outcomes, and Future Directions. J Heart Lung Transplant 2022. PMID: 35461760
- Burzotta F et al. Impella Ventricular Support in Clinical Practice: Collaborative Viewpoint from a European Expert User Group. Int J Cardiol 2015. PMID: 26363632
- Allender JE et al. Pharmacologic Considerations in the Management of Patients Receiving Left Ventricular Percutaneous Mechanical Circulatory Support. Pharmacotherapy 2017. PMID: 28741848
- Bhatt H et al. Advanced Hemodynamic Support. Springer Nature 2021. [Link is HERE]
- Stonybrook Anticoagulation Protocol with Heparin. 2019. [Link is HERE]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
Cite this article as: Salim Rezaie, "Impella Devices 101", REBEL EM blog, June 2, 2022. Available at: https://rebelem.com/impella-devices-101/.



