 Background: The first report for supplemental oxygen for angina was in 1900, and since then oxygen therapy has been a commonly used treatment of patients with ST-Elevation Myocardial Infarction (STEMI). The reason for this is the belief that supplemental oxygen will increase oxygen delivery to ischemic myocardium and help reduce myocardial injury. This belief is based off lab studies and older clinical trials, but there have been other studies that suggest potential adverse physiologic effects of supplemental oxygen in acute coronary syndromes (ACS) (i.e reduced coronary blood flow, increased coronary vascular resistance, and production of reactive oxygen species) causing vasoconstriction and reperfusion injury.
Background: The first report for supplemental oxygen for angina was in 1900, and since then oxygen therapy has been a commonly used treatment of patients with ST-Elevation Myocardial Infarction (STEMI). The reason for this is the belief that supplemental oxygen will increase oxygen delivery to ischemic myocardium and help reduce myocardial injury. This belief is based off lab studies and older clinical trials, but there have been other studies that suggest potential adverse physiologic effects of supplemental oxygen in acute coronary syndromes (ACS) (i.e reduced coronary blood flow, increased coronary vascular resistance, and production of reactive oxygen species) causing vasoconstriction and reperfusion injury.
Taking all this information together there is a lot of uncertainty over the utility of routine supplemental oxygen therapy in acute myocardial infarction. The last AHA/ACC guidelines for the management of patients with Non-ST-Elevation Acute Coronary Syndromes was published in 2014 [3]. There have been several trials published since its publication questioning several of the early treatment modalities commonly used in the pre-hospital and emergency department settings. The strength of recommendations is listed below:
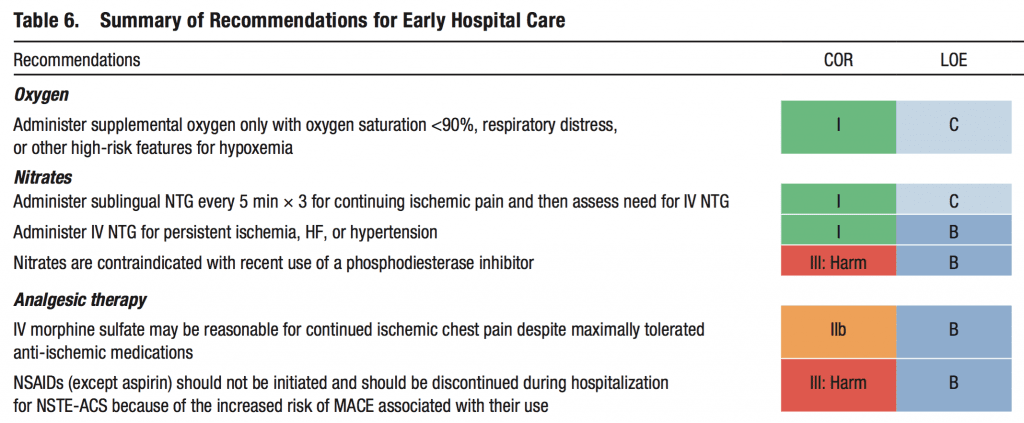
Cochrane Review 2016 [4]
- Selected randomized controlled trials in people with suspected or proven AMI (STEMI or NSTEMI within 24 hours after onset)
- Compared inhaled oxygen vs room air
- Results: 5 trials with 1173 patients
- Pooled Risk Ratio of all-cause mortality: 0.99 (95% CI 0.50 – 1.95; 4 studies, N = 1123; Low quality of evidence)
- Pooled Risk Ratio of all-cause mortality in patients with confirmed AMI: 1.02 (95% CI 0.52 – 1.98; 4 studies; N = 871; Low quality of evidence)
- Bottom Line: There is no evidence from randomized controlled trials to support the routine use of inhaled oxygen in people with AMI, and due to the low quality of evidence the harmful clinical effects of oxygen cannot be completely excluded.
AVOID Trial: Air vs O2 for STEMI [1]
What They Did:
- Compare supplemental oxygen therapy (face mask at 8 L/min) vs NO oxygen therapy in normoxic patients with STEMI (if O2 sat fell <94% received 4L/min nasal cannula or facemask 8L/min)
- Multicenter, Prospective, Open Label, Randomized Trial
- Conducted by Ambulance Victoria and 9 Metropolitan Hospitals in Melbourne, Australia
Outcomes:
- Primary Endpoint
- Myocardial injury (measured by peak cTnI and CK)
- Secondary Endpoints (At Hospital Discharge & 6 Months)
- ECG ST-segment resolution
- Mortality
- Major Adverse Cardiac Events (Death, recurrent MI, repeat revascularization, and stroke)
- Myocardial infarct size on Cardiac Magnetic Resonance Imaging (CMR) at 6 months
Inclusion:
- Age ≥ 18 years
- Chest Pain < 12 hours
- Prehospital ECG evidence of STEMI (STE of ≥0.1mV in 2 contiguous limb leads, or ≥0.2mV in 2 contiguous chest leads, or new LBBB)
Exclusion:
- Oxygen saturation <94%
- Bronchospasm requiring nebulized beta2 agonist therapy using oxygen
- Oxygen administration prior to randomization
- Altered mental status
- Planned transport to a non-participating hospital
- Deemed not to have STEMI after physician assessment at hospital
Results:
- 638 patients randomized for study à 441 with confirmed STEMI included in analysis
- Primary Outcomes:
- Mean Peak Troponin I: 57.4 mcg/L (Oxygen Group) vs 48.0 mcg/L (No Oxygen Group) [p = 0.18]
- Mean Peak CK: 1948 U/L (Oxygen Group) vs 1543 U/L (No Oxygen Group) [p = 0.01]
- Secondary Outcomes:
- Mortality at Hospital Discharge:8% (Oxygen Group) vs 4.5% (No Oxygen Group) [p = 0.11]
- In-Hospital Recurrent MI: 5.5% (Oxygen Group) vs 0.9% (No Oxygen Group) [p = 0.006]
- In-Hospital Cardiac Arrhythmias: 40.4% (Oxygen Group) vs 31.4% (No Oxygen Group) [p = 0.05]
- CMR performed on 139 patients (32%) at 6 months
- Median Infarct Size:3g (Oxygen Group) vs 13.1g (No Oxygen Group) [p = 0.04] à Expressed as proportion of left ventricular mass = 12.6% vs 9.0% respectively [p = 0.08]
Strengths:
- Multicenter, prospective randomized trial
- Large patient population
Limitations:
- This study was not powered for clinical endpoints (i.e. in-hospital recurrent myocardial infarctions, major cardiac arrhythmias, and mortality) but instead looked at surrogate cardiac biomarker and LV infarct size
- Treatment allocation not blinded to paramedics, patients or in-hospital cardiology teams, but the but 6 month follow up coordinator, statistician, and investigators undertaking data analysis were masked to treatment assignment
- There was a very low mortality rate in this trial.
- There were a lot of patients enrolled in the pre-hospital setting, who were excluded later by physicians which could cause a selection bias
- At 6-months approximately 1/3 of randomized patients had cardiac MRI, and this was due to contraindications to MRI, and availability at a single center site making it difficult for patients to travel
- There was a portion of the no oxygen arm that did receive oxygen therapy (7.7%), which may dilute the differences quoted in this study.
Discussion:
- Many believe that routine administration of supplemental oxygen in normoxic patients with STEMI reduces psychological and physiological stresses, but in this study there was no difference in chest pain scores or the requirement for additional opioid analgesics.
- Only 7.7% of patients in this study allocated to the no oxygen arm required oxygen by the time they arrived to the catheterization lab.
Author Conclusion: “Supplemental oxygen therapy in patients with ST-elevation-myocardial infarction but without hypoxia may increase early myocardial injury and was associated with larger myocardial infarct size assessed at 6 months.”
Bottom Line of AVOID Trial:
- This study demonstrated a non-statistically significant increase in myocardial infarct size with the use of supplemental oxygen with no effect on improving patient hemodynamics, or alleviating symptoms. This should be balanced with a non-statistically significant increase in mortality without the use of oxygen.
- This study was also not designed to assess the impact of lower concentrations of supplemental oxygen (i.e. <8L/min), which is what is commonly given in patients with STEMI (i.e. 2 – 4L/min)
The DETO2X-AMI Trial [2]
What They Did:
- Registry based, non-blinded, randomized clinical trial
- 6629 adult patients, aged >30 years, with symptoms suggestive of AMI for less than 6 hours with an O2 saturation > 90% and either EKG changes indicative of ischemia or a positive troponin T who presented to ambulance services, Emergency Departments (EDs), coronary care units or a catheterization laboratory at a participating hospital (35/69 Swedish Hospitals participating in the SWEDEHEART Registry)
- Supplemental oxygen (6L/min vs ambient room air)
Outcomes:
- Primary: Death from any cause at 1 year
- Secondary: Death from any cause within 30 days after randomization, rehospitalization with myocardial infarction, rehospitalization with heart failure, and cardiovascular death and composites of these endpoints at 30 and 365 days
Exclusion:
- Patients who were receiving ongoing O2 therapy, presentation with cardiac arrest or development of cardiac arrest between presentation and enrollment
Results:
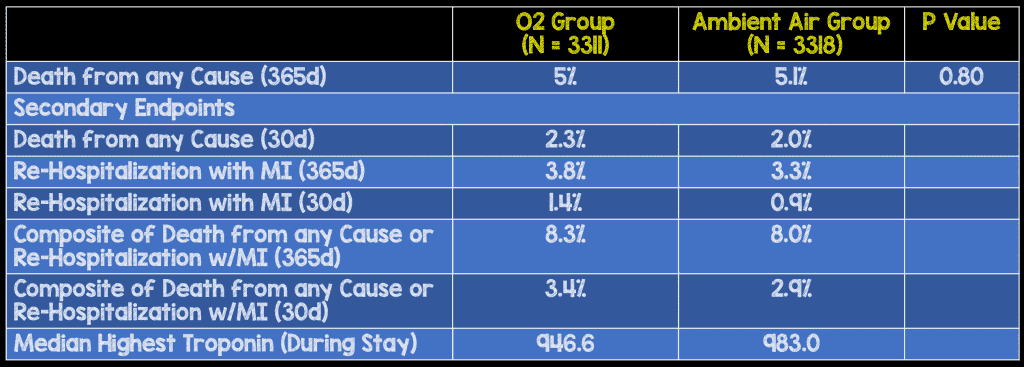
Strengths:
- The study asked a simple, clinically relevant question
- Unlike the prior AVOID trial, the endpoint is patient centered (i.e. death)
- Randomization was appropriately performed
- Most myocardial infarction related outcomes were included between the primary and secondary outcomes
- Follow up was adequate as only Swedish citizens who had a personal identification number were included so the Swedish National Population Registry could be used
Limitations:
- Blinding was not performed as pressurized air was not available at all sites
- Single country study leads to questions of external validity (homogenous population)
Author Conclusion: “Routine use of supplemental oxygen in patients with suspected myocardial infarction who did not have hypoxemia was not found to reduce 1-year all-cause mortality.”
Bottom Line of DETO2X-AMI Trial: The use of supplemental oxygen does not appear to offer any benefit to patients with acute myocardial infarction with O2 sats ≥90%
Clinical Take Home Point:
- To date there is a paucity of high quality randomized clinical trial data to support routine use of supplemental oxygen in normoxemic patients with AMI. It seems reasonable at this time, to withhold oxygen therapy in normoxemic patients
- It is important to remember that oxygen is a drug, and just like any drug we prescribe to patients it has possible significant side effects.
References:
- Stub D et al. Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation 2015. PMID: 26002889
- Hofmann R et al. Oxygen Therapy in Suspected Acute Myocardial Infarction. NEJM 2017. PMID: 28844200
- Amsterdam EA et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A Report of the American college of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am coll Cardiol 2014. PMID: 25260718
- Cabello JB et al. Oxygen Therapy for acute Myocardial Infarction. Cochrane Database Syst Rev 2016. PMID: 28595112
For More Thoughts on This Topic Checkout:
- Brent Dribble at REBEL EM: The DETO2X Trial – Do Patients with AMI Need Supplemental O2?
- July 2015 REBEL Cast: The AVOID Trial
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



