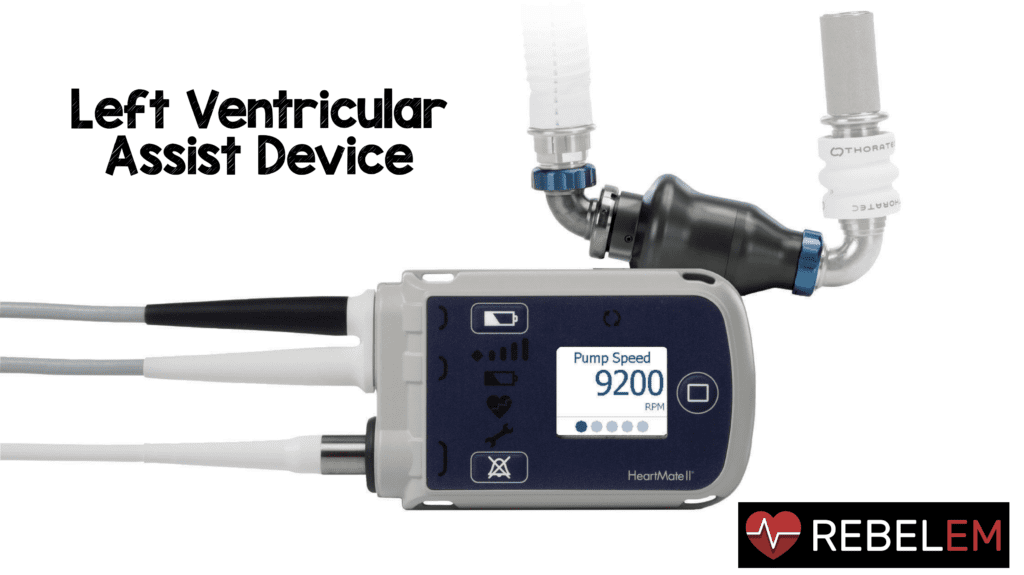
 The first left ventricular assist device (LVAD) was performed in 1984 and since that time there is an increasingly growing population of patients with LVADs. This means ED physicians will be seeing more and more of these patients in the ED and should have a basic understanding of how these devices work and have an adequate understanding of common complications and an approach to evaluate these patients. LVADs are typically used for end-stage heart failure for both a bridge to transplantion and for long-term quality of life improvement. Most of the information for this post comes from a great review article written by Chris Partyka et al in EMA 2014.
The first left ventricular assist device (LVAD) was performed in 1984 and since that time there is an increasingly growing population of patients with LVADs. This means ED physicians will be seeing more and more of these patients in the ED and should have a basic understanding of how these devices work and have an adequate understanding of common complications and an approach to evaluate these patients. LVADs are typically used for end-stage heart failure for both a bridge to transplantion and for long-term quality of life improvement. Most of the information for this post comes from a great review article written by Chris Partyka et al in EMA 2014.
What are the indications for LVAD insertion? [1]
- As a bridge to cardiac transplantation
- As a bridge to recovery (i.e. reversible myocardial pathology)
- As destination therapy = long-term treatment of patients who are not candidates for transplant
What is the one year survival rate with LVADs?
- In 2001 LVAD = 52% vs Medical Therapy = 25% [2]
- In 2013 LVAD = > 90% [3]
What are the components and types of VADs? [1]
Components:
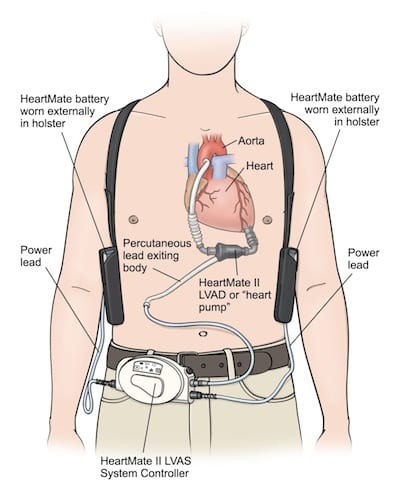 Inflow Cannula (From Left Ventricle): Pulls blood from the LV into the pump
Inflow Cannula (From Left Ventricle): Pulls blood from the LV into the pump- The Pump: generates blood flow
- Outflow Conduit (Anastomosed to Ascending Aorta): Pushes blood into the aorta
- Driveline (Percutaneous Lead): Connection to external controller
- External System Components (Controller, Monitors, Power Source, and Battery): Monitors VAD performance, displays battery life, and typically two batteries
Types:
- Left Ventricular Assist Device (LVAD)
- Right Ventricular Assist Device (RVAD)
- Biventricular Assist Device (BiVAD)
- Pulsatile Flow Devices
- Continuous Flow Devices
What is the physiology of continuous flow devices? [1]
Increased diastolic pressure and flow allowing for continuous flow of the entire cardiac cycle, meaning aortic flow is present during diastole when normally absent. This results in a diminished pulse pressure. Also LVADs are preload dependent (i.e. they only pump blood or volume that gets to it) so maintenance of preload is of utmost importance.
What does the pump of a pulsatile vs continuous flow VAD look like?[4]
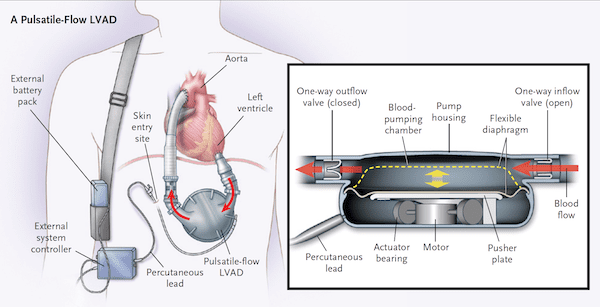
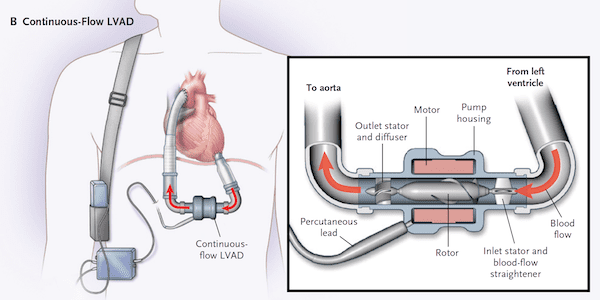
How do you assess VAD performance? [1]
- The first thing to remember is peripheral and central pulses may be diminished or absent, non-invasive blood pressure measurements are difficult to obtain.
- The 12 lead ECG can give you your heart rate and should be performed on all patients with VADS
- BP can be documented by blood pressure cuff and doppler ultrasound over brachial or radial artery
- In critically ill patients, BP can be documented by an arterial line
Many patients with VADs can present with hypotension. What are some of the causes of this?[1]
Remember this can be a normal finding in patients with VADs, but it is important to go through a systematic differential as you evaluate.
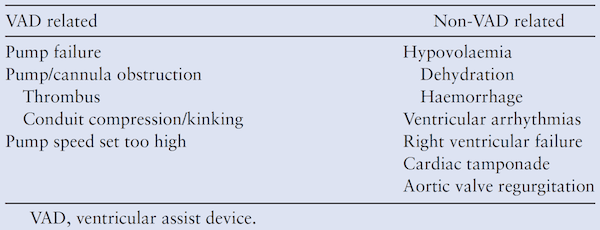
How do you assess VAD function? [1]
- You can tell if the pump is running simply by placing your stethoscope over the precordium or epigastrium and listen for a continuous machinery like noise
- The external controller will tell you some important information as well: pump speed (rpm), flow (L/min), power (watts), and remaining battery life
- Normal values:
- Pump Speed: 2200 – 2800 rpm (HeartWare VAD) and 8000 – 10000 rpm (HeartMate II VAD)
- Power: 4 – 6 Watts
- Flow: 4 – 6 L/min
- Pulsatility Index (PI): 1 – 10
How often does life threatening VAD malfunction occur? [1]
- 1st generation VADs: 6% at 6 months and 64% at 2 years
- 2nd generation VADs: 54% at 2 years for continuous flow LVAD [cite source=”pubmed”]19920051[/cite]
What are some of the common VAD malfunctions, how should they be evaluated, and treated?[1]
- Always stabilize the patient first, but with the first opportunity you get, contact the local VAD-coordination team or center for assistance and/or to transfer the patient to a VAD center!!!
- Pump Thrombus:
- Occurs in 1 – 2% of patients at 2 years post implantation resulting in low cardiac output
- Echo and cardiac CT can aid in diagnosis
- Treatment is immediate anticoagulation with unfractionated heparin, and in worst case scenarios (i.e. coding) thrombolytic therapy
- Suction Events:
- With negative pressure in the left heart, the left ventricle can collapse from leftward displacement of the septum (aka “suction event”)
- This can be due to a whole host of reasons including: hypovolemia, cardiac tamponade, arrhythmias, and malposition of the inflow tract.
- Treatment should begin with IVF to maintain preload but ultimately these patients should be transferred to VAD-specialist center and if there is an issue with the inflow tract, management typically requires surgical exploration
- Bleeding:
- Typically non-surgical and one of the most common reasons to present to the ED
- The reason for this is multifactoral:
- Patients with VADs are on anticoagulation and anti platelet therapy to reduce risk of thromboembolic events
- Patients often get an acquired von Willebrand syndrome from the shear forces created by the VAD itself
- Reduced pulse pressure results in arteriovenous malformations.
- Treatment may require reversal but also puts these patients at risk for thrombosis and stroke. Consider tranexemic acid, DDAVP, PCCs, recombinant factor VII, and FFP only after discussion with a VAD specialist.
- RV Failure:
- This occurs in about 20 – 25% of patients and typically occurs in the early few days post VAD implantation.
- Echo is a useful modality for diagnosis.
- Treatment is complex including inotropic support, volume unloading and pulmonary vasodilators.
- Arrhythmias:
- Incidence can be as high as 50%, but fortunately the treatment of these is no different than patients without VADs.
- Treatment of Ventricular Arrhythmias: Consider IV amiodarone as first line agent, but lidocaine and procainamide can be considered as second line agents
- Cardioversion/Defibrillation is best performed with an anterior-posterior placement of pads. Some VADs may be able to internally defibrillate.
- Infection:
- Infection with VADs can include the surgical site, driveline, device pocket, and/or the pump itself.
- If you suspect infection, again the treatment for this is no different than early IVF and early antibiotics. The only difference is these patients should be transferred to the nearest VAD facility for more definitive treatment (i.e. VAD replacement, etc…).
- Neurologic Complications and Stroke: Remember these patients have a higher risk of hemorrhagic stroke due to the reasons mentioned above. This can occur in up to 27% of patients with VADs.
- Cardiac Arrest:
- Again ABC’s are paramount. Intubation, IVF, and assessment of the VAD itself as outlined above.
- Echo can be very helpful to assess RV and LV size and function.
- CPR should be performed only if absolutely necessary, but remember this might lead to damage of the VAD itself. There was a small retrospective case series of 8 patients with VADs who received CPR during cardiac arrest, which showed no dislodgment or damage to the VADs, and 4 of the 8 patients surviving with neurologically good outcomes, but larger trials are needed before making this the standard practice. [5]
Take Home Messages:
- Always contact the closest VAD coordinator or cardiac transplant center as soon as possible
- Hypotension can be a non-specific finding with VADs but always look for other etiologies
- VADs are preload dependent
- Check an ECG on all patients who have VADs
References:
- Partyka C et al. Review Article: Ventricular Assist Devices in the Emergency Department. Emerg Med Australas 2014. PMID: 24707998
- Rose EA et al. Long-Term use of a Left Ventricular Assist Device for End-Stage Heart Failure. NEJM 2001. PMID: 11794191
- Wever-Pinzon O et al. Morbidity and Mortality in Heart Transplant candidates Supported with Mechanical Circulatory Support: Is Reappraisal of the Current United Network for Organ Sharing thoracic Organ Allocation Policy Justified? Circ 2013. PMID: 23271796
- Slaughter MS et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. NEJM 2009 PMID: 19920051
- Shinar Z et al. Chest Compressions May be Safe in Arresting Patients with Left Ventricular Assist Devices (LVADs). Resus 2014. PMID: 24472494
For more on this topic check out:
- Chris Partyka on his blog The Blunt Dissection
- This Post Also Featured on ALiEM Cardiovascular Approved Instructional Resources (AIR) Series (2015)
- CanadiEM: Mechanical Heart – A Basic Approach to LVADs in the ED



