 Background: Normal Saline (NS) is one of the most commonly used IVFs in resuscitation today. The use of balanced vs unbalanced crystalloids has been one of the biggest debates in resuscitation of the critically ill in recent history due to concerns of unbalanced fluids causing acute kidney injury, hyperchloremic metabolic acidosis, and worsened mortality. In 2015, we saw the publication of the SPLIT trial, which we covered on REBEL EM. This was a randomized clinical trial of over 2200 patients in 4 ICUs in New Zealand comparing 0.9% Saline (NS) vs Plasma-Lyte. This trial had many issues including, >70% of patients coming from the OR, only 15% came from the ED, only 4% had sepsis, and the biggest issue with this trial was that the majority of patients only received 1 – 2L of NS, making it unclear if larger volumes of unbalanced crystalloid would have worsened morbidity and mortality. Since the publication of this study, two more trials have been published: The SALT-ED Trial and The SMART trial (Both Just published in the NEJM Feb 27th, 2018).
Background: Normal Saline (NS) is one of the most commonly used IVFs in resuscitation today. The use of balanced vs unbalanced crystalloids has been one of the biggest debates in resuscitation of the critically ill in recent history due to concerns of unbalanced fluids causing acute kidney injury, hyperchloremic metabolic acidosis, and worsened mortality. In 2015, we saw the publication of the SPLIT trial, which we covered on REBEL EM. This was a randomized clinical trial of over 2200 patients in 4 ICUs in New Zealand comparing 0.9% Saline (NS) vs Plasma-Lyte. This trial had many issues including, >70% of patients coming from the OR, only 15% came from the ED, only 4% had sepsis, and the biggest issue with this trial was that the majority of patients only received 1 – 2L of NS, making it unclear if larger volumes of unbalanced crystalloid would have worsened morbidity and mortality. Since the publication of this study, two more trials have been published: The SALT-ED Trial and The SMART trial (Both Just published in the NEJM Feb 27th, 2018).
The SALT-ED Trial [1]
What They Did:
- Single-center, pragmatic, unblinded, multiple-crossover trial comparing balanced crystalloids (LR or Plasma-Lyte A) vs NS among adults treated with IVF in the ED and admitted to the hospital outside the ICU
- IVF alternated monthly between NS and balanced crystalloids (LR or Plasma-Lyte A)
- Saline Against Lactated Ringer’s or Plasma-Lyte in the Emergency Department (SALT-ED)
Outcomes:
- Primary: Hospital-Free Days (Days alive after discharge before day 28) – Composite of in-hospital death and hospital length of stay (Number of days alive and out of the hospital between the index ED visit and 28 days)
- Secondary:
- Major Adverse Kidney Events at 30d (MAKE30) – composite of death from any cause, new renal-replacement therapy or persistent renal dysfunction
- In-hospital mortality = Death prior to hospital discharge
- New receipt of RRT = Receipt of any modality of RRT prior to hospital discharge in a patient not known to have received RRT prior to ICU admission.
- Persistent renal dysfunction = Final plasma creatinine value before hospital discharge ≥ 200% of the baseline plasma creatinine value in a patient not known to have received RRT prior to ICU admission.
- Acute kidney injury of stage 2 or higher
- In-hospital mortality
- Major Adverse Kidney Events at 30d (MAKE30) – composite of death from any cause, new renal-replacement therapy or persistent renal dysfunction
Inclusion:
- Adults ≥18 years of age
- Received at least 500cc of IV isotonic crystalloids in the ED
- Admitted to the hospital outside the ICU (ICU admitted patients included in the SMART study)
- Patients with ESRD on HD were not eligible to meet renal outcomes (New RRT, persistent renal dysfunction, and AKI), but could meet MAKE30 within 30 days
Exclusion:
- Patients receiving <500cc of IV isotonic crystalloids in the ED
Results:
-
- BC = 6708 patients
- NS = 6639 patients
- Median Crystalloid Volume Received = 1079mL
- ≥2000mL Crystalloid Volume Received ≈ 33%
- 88.3% of patients exclusively received assigned crystalloid13,347 patients enrolled in final analysis
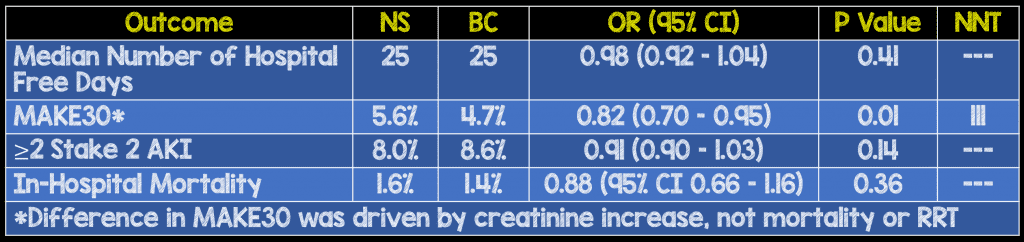
Components of the MAKE30 Composite Outcome
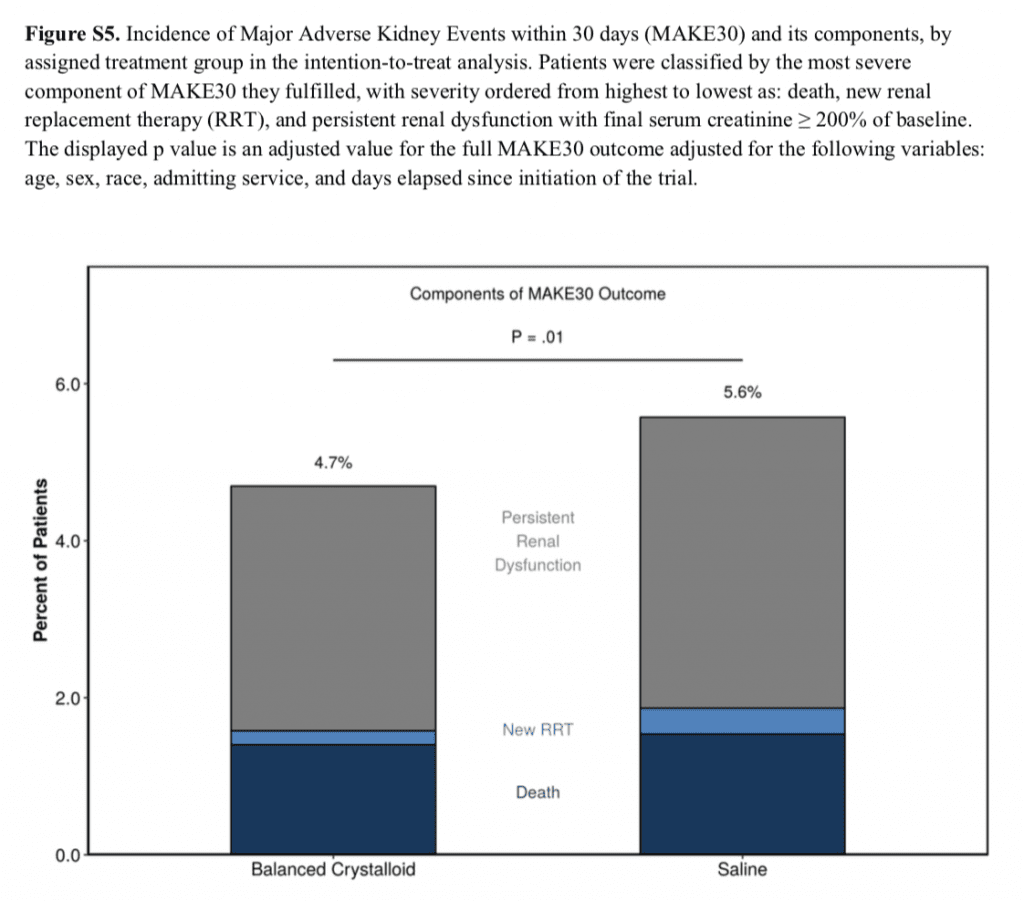
Strengths:
- Large, pragmatic randomized trial
- Design and statistical analysis plans were prespecified
- Trial population was limited to only 1st ED visit (i.e. only unique patients included)
- Baseline characteristics were similar between groups
- Majority of patients received per-protocol crystalloids (83.8% in BC and 92.8% in NS groups)
Limitations:
- Single center
- Physicians and patients were not blinded to the type of fluid received – this could cause biases in the results either diluting or magnifying the results
- Providers had the option of ordering off-protocol crystalloids and these patients were included in the primary analysis which could dilute the results of the study. However, there was a per-protocol analysis which included only patients who received fluids in accordance with the protocol
- Majority of BC received was LR (95.3%) making it difficult to draw conclusions about Plasma-Lyte A
- Data collection from an EMR did not allow for more detailed information about patient characteristics
- Fluids administered after hospital admission and those used as medication carriers were not controlled for in this trial
Discussion:
- Most Common Admission Services:
- General Medicine ≈ 71%
- General Surgery ≈ 19.1%
- Cardiology ≈ 5%
- Patients with baseline serum Cr ≥1.5mg/dL or serum Cl >110mmol/L appeared to have largest benefit from BC for avoiding MAKE30 and AKI
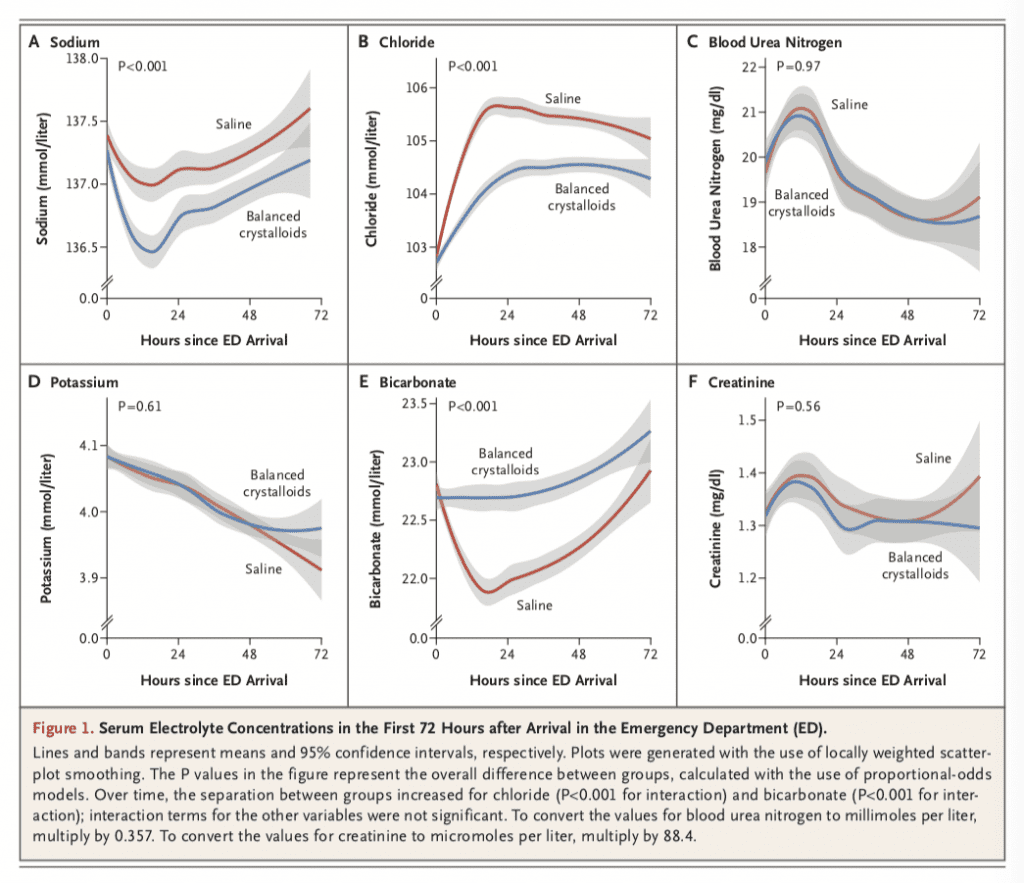
- Non-Patient Oriented Outcomes (i.e. Electrolytes)
Author Conclusion: “Among noncritically ill adults treated with intravenous fluids in the emergency department, there was no difference in hospital-free days between treatment with balanced crystalloids and treatment with saline.”
SALT-ED Clinical Take Home Point:
- In non-critically ill adults admitted to the hospital, excluding ICU admissions, from the ED there was no difference in hospital-free days.
- A secondary outcome demonstrated a statistically significant decrease in MAKE30 with use of balanced crystalloids compared to NS with NNT = 111. This result was primarily driven by doubling of creatinine, not mortality or need for RRT.
- Patients with baseline serum Cr ≥1.5mg/dL or serum Cl >110mmol/L appeared to have largest benefit from BC for avoiding MAKE30 and AKI.
- This data is very promising and the current best evidence to use balanced crystalloids, but it is unblinded (a huge methodological issue). A properly blinded RCT should be the next step in this current debate of balanced vs unbalanced crystalloids, but until that time, if you haven’t already….its time to switch to balanced crystalloids
The SMART Trial [2]:
What They Did:
- Pragmatic, unblended, cluster-randomized, multiple-crossover trial in 5 ICUs
- 15,802 adults randomized to NS or BC (LR or Plasma-Lyte A)
- Isotonic Solutions and Major Adverse Renal Events Trial (SMART)
Outcomes:
- Primary: Major Adverse Kidney Event at day 30 (MAKE30) – Composite of death from any cause, new renal-replacement therapy, or persistent renal dysfunction
- In-hospital mortality = Death prior to hospital discharge
- New receipt of RRT = Receipt of any modality of RRT prior to hospital discharge in a patient not known to have received RRT prior to ICU admission.
- Persistent renal dysfunction = Final plasma creatinine value before hospital discharge ≥ 200% of the baseline plasma creatinine value in a patient not known to have received RRT prior to ICU admission.
- Secondary:
- In-hospital mortality before ICU discharge or at 30d or 60d
- ICU-free days
- Ventilator-free days
- Vasopressor-free days
- Days alive and free of RRT during 28 days after enrollment
Inclusion:
- Adults ≥18 years of age
- Admitted to ICU
Exclusion:
- Age <18 years
Results:
- Enrolled Patients
- BC = 7942 patients
- NS = 7860 patients
- >1/3 on mechanical ventilation
- >1/4 on vasopressors
- Cumulative Volume Through Day 3
- NS = 1935 +/- 2873mL
- BC = 1872 +/- 2744mL
- Median Volume Administered
- BC = 1000mL (Range 0 – 3210)
- NS = 1020mL (Range 0 – 3500)
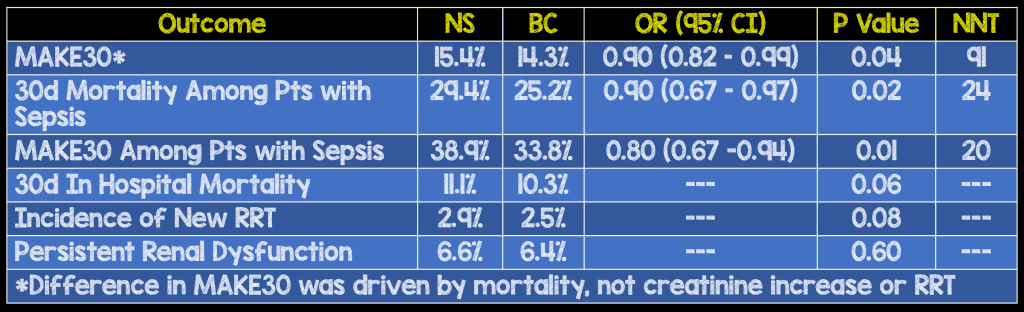
Components of the MAKE30 Composite Outcome
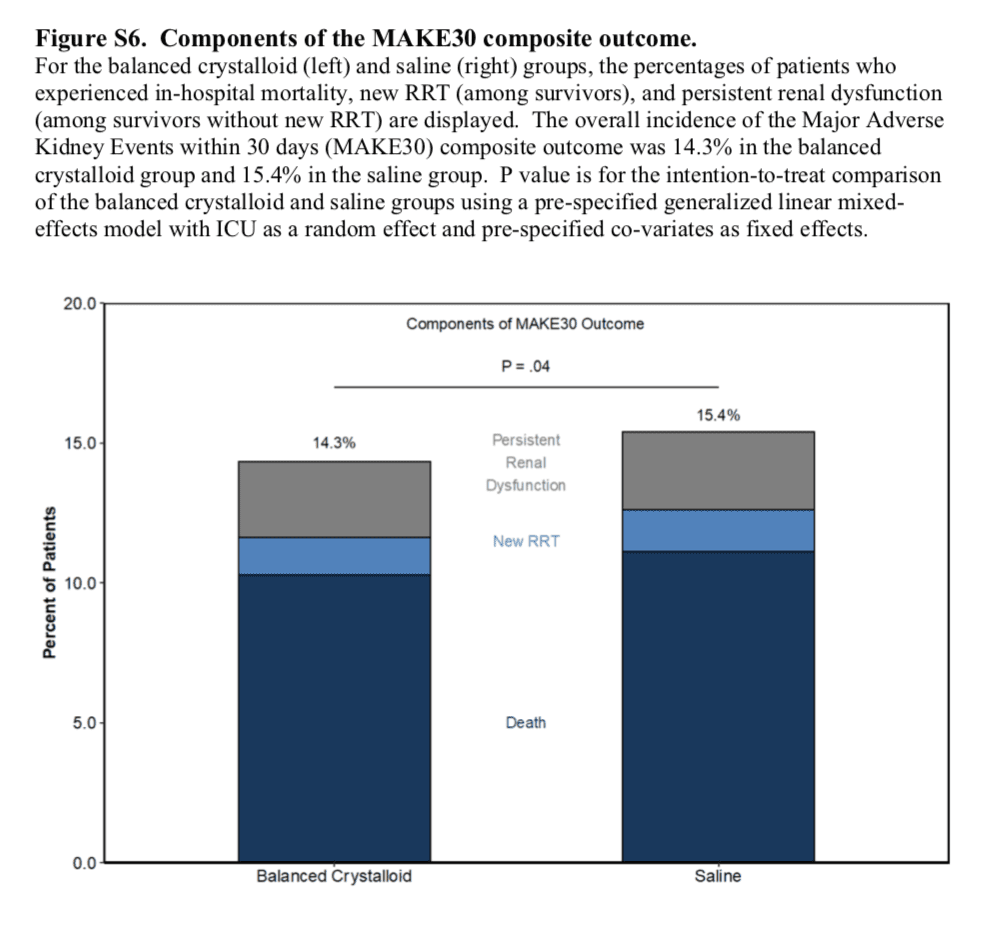
- No difference in ICU-free days, ventilator-free days, vasopressor-free days, RRT-free days
Strengths:
- Large, pragmatic, randomized trial, which allowed statistical power to detect small differences in patient outcomes
- Clinically important question
- Group assignments occurred at the level of the ED, this allowed delivery of the assigned fluid early in each patients care minimizing selection bias and improved generalizability of the results
- Protocol and statistical analysis plans were published before the conclusion of enrollment
- Methodologically interesting that the authors set a target for their primary outcome, but then reset it based on observational data. This is really nice work by the authors to make sure the study was done right and led to a larger enrollment for statistical power
- No baseline differences between groups
Limitations:
- Single academic center
- Enrolled patients who were discharged from the hospital were eligible to participate again if readmitted to the ICU…this could potentially dilute or magnify results
- Patients, clinicians, and investigators were aware of group assignments which could bias the results (i.e. unblinded study). Death and creatinine level are objective results, however the decision to initiate renal replacement therapy is subjective and susceptible to treatment bias
- LR and Plasma-Lyte A were evaluated together, therefore this trial does not inform choice between these to BCs
- The need for IVF at all times did not allow for washout periods (i.e. Patients who were in the ICU at the end of one calendar month to the start of the next may have been exposed to both types of crystalloids diluting the results of the trial) – only 4.4% of patients in the NS group and 5.4% in the BC group.
Discussion:
- In patients with traumatic brain injury (TBI), clinicians had the option of using 0.9%NS instead of BC regardless of trial group. Therefore the results of this study cannot provide guidance whether to use NS or BC in patients with TBI.
- Hyperkalemia was used as a relative contraindication to use balanced crystalloids. It should be noted that this is a non-sensical recommendation.
- The most common source of admission to the ICU was as follows:
- ED ≈ 50%
- OR ≈ 21%
- The most common ICU admission diagnoses were as follows:
- Sepsis or Septic Shock ≈ 15%
- TBI ≈ 9%
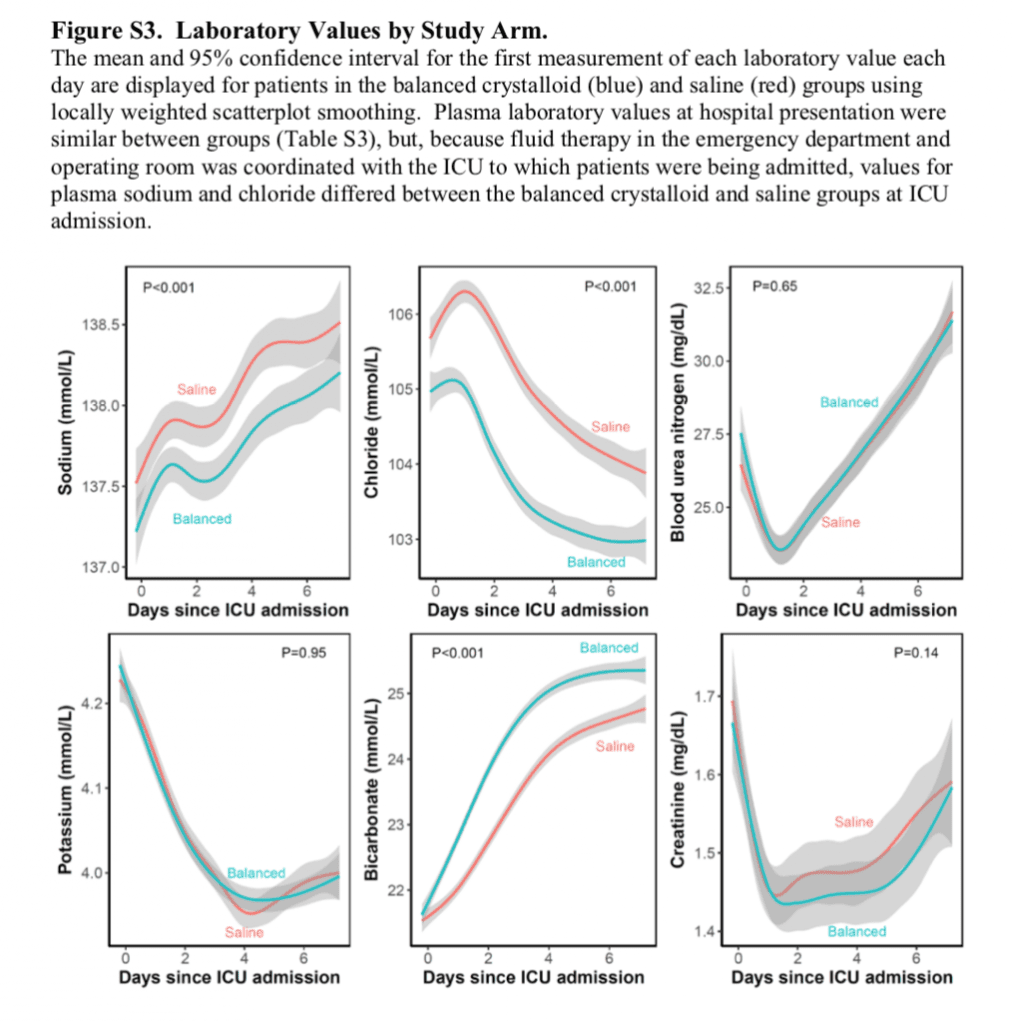
- Non-Patient Oriented Outcomes (i.e. Lab Values)
Author Conclusion: “Among critically ill adults, the use of balanced crystalloids for intravenous fluid administration resulted in a lower rate of the composite outcome of death from any cause, new renal-replacement therapy, or persistent renal dysfunction than the use of saline.”
Clinical Take Home Point: In critically ill patients, excluding TBI patients, admitted to the ICU, the use of BC resulted in lower MAKE30. The largest benefit appears to be in the septic subset of patients with a NNT of 20.
Myburgh Editorial [3]:
In his editorial John Myburgh comments:
- Use of balanced crystalloids significantly reduced MAKE30 (Composite of Death from any cause, New RRT, or Persistent renal dysfunction) in both trials.
- Composite outcomes are often used in trials to mitigate competing risks in trials with low event rates, such as the two trials reviewed in this post.
- The composite outcome in these trials were driven by reduced persistent AKI at day 30 and not short-term mortality or use of RRT. (MY REBUTTAL TO THIS: This does not appear to be the case in the critically ill population, especially in septic patients).
- Death, new RRT, and doubling of creatinine are not equivalent outcomes when taken individually.
- The two trials reviewed do not give us assessments of longer-term patient centered outcomes and health economics
- Caution is required interpreting the results of these trials as it does not provide unequivocal clinical evidence for balanced fluids
Clinical Bottom Line:
- There is nothing normal about Normal Saline
- Based on the available evidence it is impossible to determine a difference between balanced crystalloids (i.e. LR vs Plasma-Lyte A)
- The results of these studies DO NOT provide guidance whether to use NS or BC in patients with TBI, although I would argue that both are safe
- There are some methodological issues in both trials, as are present in many trials, however despite these issues it appears that based on these two large, randomized trials, that we now have the strongest evidence to date supporting the use of balanced crystalloids over normal saline in both critically ill and non-critically ill patients admitted to the hospital
References:
- Self WH et al. Balanced Crystalloids Versus Saline in Noncritically Ill Adults. NEJM 2018 [Epub Ahead of Print]
- Semler MW et al. Balanced Crystalloids Versus Saline in Critically Ill Adults. NEJM 2018. [Epub Ahead of Print]
- Myburgh J. Patient-Centered Outcomes and Resuscitation Fluids. NEJM 2018 [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- Josh Farkas at PulmCrit (EMCrit): Get SMART – Nine Reasons to Quit Using Normal Saline for Resuscitation
- REBEL EM: The SPLIT Trial – Saline vs Plasma-Lyte Fluid Therapy
- Rory Spiegel at EMNerd (EMCrit): The Case of the Unbalanced Solution
- Fraser Magee at The Bottom Line: SALT-EM
- Celia Bradford at The Bottom Line: SMART
- Simon Carley at St. Emlyn’s Blog: JC – Balanced Fluids s Saline on the ICU. The SMART Trial
- Dan Horner at St. Emlyn’s Blog: JC – So Long Salt and Saline?
- The Resus Room: The Crystalloid Debate
- Justin Morgenstern at First10EM: IV Fluid Choice Part 2 – The SMART Trial
- Justin Morgenstern at First10EM: IV Fluid Choice Part 3 – The SALT-ED Trial
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



