Article: Murai IH et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021. PMID: 33595634 [Open in Read by QxMD]
Clinical Question: What is the effect of a single, high dose of vitamin D3 on hospital length of stay among patients with moderate to severe coronavirus disease 2019 (COVID-19)?
Population: Patients aged > 18 years of age who tested positive by PCR or computed tomography scan findings compatible with the disease (bilateral multifocal ground-glass opacities ≥50%); and diagnosis of flu syndrome with institutional criteria for hospitalization like respiratory rate greater than 24/min, saturation less than 93% while breathing room air, or risk factors for complications (eg, heart disease, diabetes, systemic arterial hypertension, neoplasms, immunosuppression, pulmonary tuberculosis, obesity) followed by COVID-19 confirmation on hospital admission at two hospitals in Sao Paulo, Brazil.
Intervention: Single oral dose of 200,000 IU of vitamin D3 dissolved in a 10-mL peanut oil solution
Control: Placebo group received 10 mL of a peanut oil solution
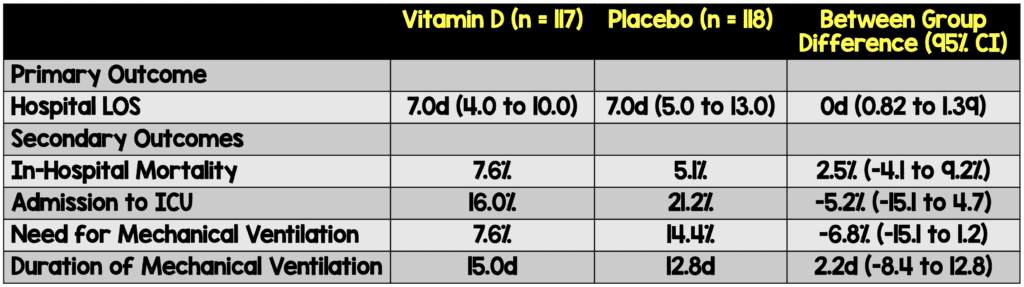
Primary Outcomes: Hospital length of stay defined as total number of days patient remained hospitalized from the date of randomization. Criteria for discharge were no need for supplemental oxygen in the past 48 hours, no fever in the past 72 hours, and oxygen saturation greater than 93% without supplemental oxygen and without respiratory distress
Secondary Outcomes: Mortality, Patients admitted to ICU, Patients on mechanical ventilation and duration.
Design: Multicenter, double-blind, parallel-group, randomized, placebo-controlled trial
Excluded: Patients were excluded if they were unable to read and sign the written informed consent form, were already admitted and receiving invasive mechanical ventilation, received previous vitamin D3 supplementation (>1000 IU/d), had kidney failure requiring dialysis or creatinine of at least 2.0 mg/dL, had hypercalcemia (total calcium >10.5 mg/dL), were pregnant or lactating, or had expected hospital discharge in less than 24 hours.
Results:
- Study assessed 1240 patients
- 1000 excluded
- 240 patients enrolled
- 117 Received Vitamin D
- 118 Received Placebo
Critical Findings:
Strengths:
- Randomized, double blind, placebo controlled experimental design
- Patients enrolled in the study had low attrition rate
- Only 1.25% of patients had missing data; fantastic follow up
- Clinically important question
- Significant racial mix of patients, increased generalizability.
Limitations:
- Fairly small study in just two hospital locations in one city, may have been underpowered to find a statistically or clinically meaningful difference
- Generalizability of results due to single location
- 1000 patients excluded from study, thus results applicable to only small subset of patients
- Vitamin D group had higher BMI
- HTN and DM were more common in the Vitamin D group. These are known risk factors for worse outcomes and may have biased the data
- No mention of standard therapies given to patients in either group
Discussion:
- In this well done randomized double blind placebo controlled trial the researchers evaluated to see if there was any relationship between Vitamin D3 administration and improved outcomes which, unfortunately, there was not.
- The trial did manage to show an elevation in patients 25-hydroxyvitamin D levels however there was no clinical difference between the patients with elevation and those without.
- This study demonstrated that though Vitamin D is theorized to regulate both innate and adaptive immune responses there appeared to be no clinical benefit to have elevated levels in patients.
- While not shown to have a significant difference, there was a signal of benefit in patients that required admission to the ICU and required mechanical ventilation. These were both subjective secondary outcomes and the study wasn’t powered to identify significant differences in them. Further studies should be conducted to evaluate for potential difference
- Using the same reasoning we see that placebo had lower in hospital mortality than Vitamin D. We cannot make conclusions based on these findings because again, the sample size was small and did not reach a significant difference.
Authors Conclusions: “Among hospitalized patients with COVID-19, a single high dose of vitamin D3, compared with placebo, did not signifi- cantly reduce hospital length of stay. The findings do not support the use of vitamin D3 for treatment of moderate to severe COVID-19..”
Our Conclusions: We agree with the authors; based on this data set, there does not appear to be a role for Vitamin D3 for decreasing hospital length of stay in COVID-19.
Potential to Impact Current Practice: No impact at all
Bottom Line: Vitamin D supplementation should not be standard care in hospitalized patients with COVID-19. Further clinical trials should look to study utility in those with vitamin D deficiency and earlier use of the medication.
References:
- Murai IH et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021. PMID: 33595634 [Open in Read by QxMD]
For More on This Topic Checkout:
- LITFL: Vitamin D in Critical Illness
- REBEL EM: Does D Stand for Decrease Deterioration and Death? The Jury is Out for Vitamin D in COVID
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)



