Article: Kumar M et al. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology. 2020;71(1):235-246. PMID: 31148204
Clinical Question: Does a TEG-guided transfusion strategy lead to lower use of blood products compared with standard practice (guided by PT and INR) in acute non-variceal bleeding among patients with advanced cirrhosis?
What They Did
- Single-center randomized controlled study design performed by the Department of Hepatology & Liver transplantation in New Delhi
- Study participants, investigator clinicians, data collectors, and data analysts were blinded
- Investigators recruited participants from February 27, 2016 – March 3, 2018.
- Patients were randomized to a transfusion strategy guided by TEG or SOC 1:1
- INR, PLT count, fibrinogen every 8 hours; subsequent corrections made
- Recorded amount of blood components transfused, transfusion-related side effects, failure to control bleeding or prevent rebleeding, and duration of hospital stay
- Followed up for 6 weeks after discharge
Population
- Inclusions:
- Patients with advanced liver cirrhosis of any etiology
- Age 18-80 years
- Confirmed nonvariceal upper GI bleeding—endoscopy revealing ongoing bleeding from a nonvariceal source.
- Significant coagulopathy: INR > 1.8 and/or PLTs < 50 x10^9/L
- Exclusions:
-
-
- Variceal bleed
- Post-variceal ligation ulcer bleed
- Previous or current thrombotic events
- Any documented blood clot in a venous or arterial vessel
- Antiplatelet or anticoagulant therapy at the time of enrollment or any that had been discontinued < 7 days before the study
- Hemodialysis in the previous 7 days
- Pregnancy
- Significant cardiopulmonary disease
-
-
Intervention
- TEG group:
- R time > 10 minutes: patients received FFP at 10 cc/kg of IBW
- MA < 55 mm: patients received single donor apheresis platelet unit (6-8 pooled units of platelets)
- Alpha angle < 45 degrees: patients received Cryoprecipitate (5 pooled units)
Control
- SOC group:
- INR >1.8: patients received FFP at 10 cc/kg of IBW
- PLT < 50 x 10^9/L: patients received single donor apheresis platelet unit
- Fibrinogen < 80 mg/dL: patients received cryoprecipitate 5 pooled units
Outcomes
- Primary Outcome:
- Amount of FFP transfused in milliliters
- Secondary Outcomes:
- Five day treatment failure (failure to control bleed): death or need to change therapy
- Fresh hematemesis or nasogastric aspiration at least 100 mL of fresh blood 2 hours or more after the start of a specific drug treatment or therapeutic endoscopy
- Development of hypovolemic shock
- 3g drop in hemoglobin (9% drop of hematocrit) within any 24 hour period if no transfusion administered
- Failure to prevent rebleeding after 5 days: single episode of clinically significant rebleeding
- Recurrent melena or hematemesis resulting in
- Hospital admission
- Blood transfusion
- 3g drop in hemoglobin
- Death within 6 weeks
- Recurrent melena or hematemesis resulting in
- Amount of platelets and cryoprecipitate transfused
- Transfusion-related reactions
- Duration of ICU and hospital stay
- Survival at 6 weeks
- Five day treatment failure (failure to control bleed): death or need to change therapy
Results
- 397 patients with advanced cirrhosis with upper GI bleed screened
- 301 patients excluded
- 96 patients with non-variceal bleeds
- 49 patients allocated to the TEG group
- 47 patients allocated to the SOC group
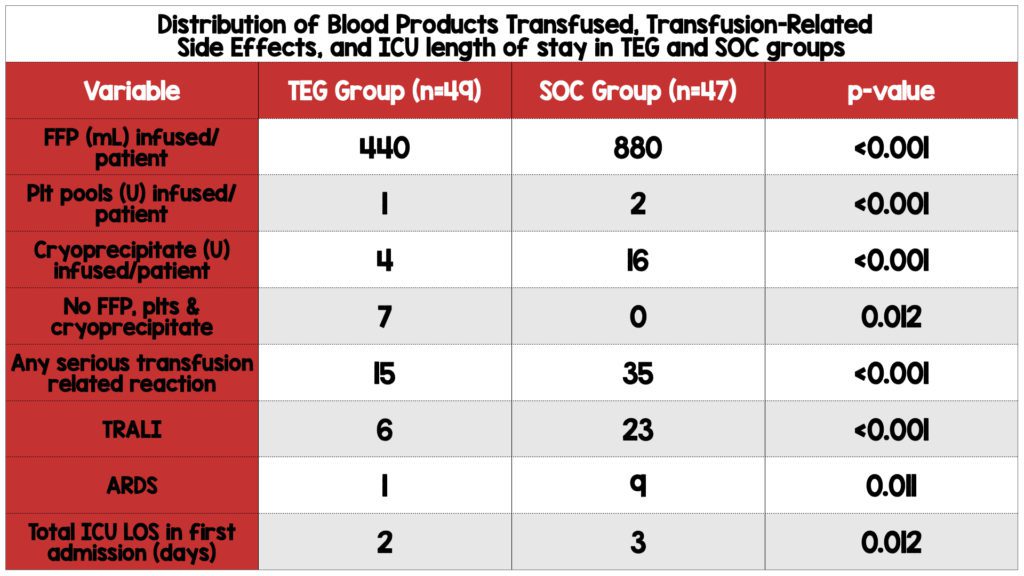
- The TEG group required less FFP, platelet, and cryoprecipitate transfusions and had a lower frequency of transfusion-related reactions compared to the SOC group.
- All patients in the SOC group required transfusion of products, while seven patients in the TEG group did not require any.
- There was no statistically significant difference in the number of red blood cells transfused between the two groups.
- The TEG group had a shorter ICU length of stay in the first admission.
- There was no statistically significant difference in failure to control bleeding by day 5 or failure to prevent rebleeding after day 5, total hospital length of stay in the first admission, total ICU and hospital length of stay up to 42 days, or 5 and 42-day mortality rates between the two groups.
Strengths
- Participants, investigator clinicians, data collectors, and data analysts were blinded.
- Randomized study design
- Blind adjudication of transfusion reaction outcomes
- Follow-up was complete.
- Investigators performed an Intention-to-treat analysis.
- Patients from both groups are well balanced regarding results from a wide range of coagulation studies.
- Patients from both groups had relatively high MELD scores 21-23 and severe coagulopathy (INR 2.5 and 2.6, Plt 37×10^9/L and 40×10^9/L, SOC and TEG, respectively)
- All patients enrolled had an upper endoscopy and verified the source of bleeding.
Limitations
- Single center study in India
- A very specific subset of patients with advanced liver cirrhosis and non-variceal upper GI bleeding (gastritis, portal hypertensive gastropathy, ulcers)
- Investigators also excluded patients on antiplatelet and anticoagulant therapy, likely eliminating many patients with diabetes and coronary artery disease.
- Patients exclusively managed in the ICU which decreases applicability for patients in other locations
- Very small sample size of 96 patients
- No definition was provided for exclusion criteria of significant cardiopulmonary disease.
- The primary outcome was disease-oriented
- The enrolled cohort appears to be a convenience sample
- Sealed envelopes are a weak type of allocation and prone to error and manipulation.
- The groups are not matched regarding some demographic, including Hep B, Hep C, use of beta blocker, and prior history of bleeding.
- No data was provided on whether endoscopic treatments or medical interventions were performed, contributing to co-intervention bias.
Discussion
- Study Population:
- The enrolled cohort of patients appears to be a convenience sample. It’s impossible to know whether eligible patients who were not enrolled carry the same risk as patients in the study. Furthermore, the study population represents a specific cohort of cirrhotic patients with nonvariceal bleeding. EGD results are likely unavailable to practitioners outside the ICU, limiting the application of the data. Likewise, investigators also excluded patients on antiplatelets and anticoagulants, further limiting the generalizability of results.
- Table 1 Analysis:
- Simply put, Table 1 defines “Who is in this study?” (Hayes-Larson 2019). It provides demographic information on the study sample to enable the reader to assess threats to internal and external validity, such as confounding, selection bias, and measurement error. As described in the CONSORT statement, the use of p-values in table 1 is inappropriate as the study is not powered to show a difference in baseline characteristics between the treatment and control group in an RCT (Schulz 2010). In this case, this study is solely powered to evaluate if there is a difference in the amount of FFP transfused between the two groups.
- In fact, there is a higher proportion of males and cirrhosis secondary to NASH in the SOC group. While in the TEG group, there is a higher proportion of patients with Hep B and Hep C cirrhosis, grade 2 hepatic encephalopathy, prior bleeding episodes, and beta-blocker use. It’s unclear whether these differences are clinically relevant or had any bearing on the study outcome.
- Bias:
- The resident managing patients in the ICU was unblinded. There is no information on interventions or medical therapies outside the transfusion strategy. However, patients with bleeding ulcers commonly receive other interventions, i.e., proton pump inhibitors. The care team could have provided more aggressive medical management outside the transfusion strategy to one arm over another, leading to co-intervention bias.
- Furthermore, endoscopies can be both diagnostic and therapeutic. It’s unclear whether interventions such as cauterization, clip placement, epinephrine injection, etc., were performed. If these were done, were there any protocols that the providers were following? Likewise, were there any differences in interventions between the two groups? Were these providers blinded? If not, could one group have received more aggressive management than another?
- Inside The Numbers:
- Patients in the TEG group received half as much FFP and a third of the amount of platelets and cryoprecipitate required compared to the SOC group. Furthermore, 14.3% of patients in the TEG group did not require transfusion of any blood products, while all patients in the SOC group required transfusion. Despite the lower transfusion requirements in the TEG group, rates of rebleeding, hospital and ICU length of stay, and mortality rates were similar between the two groups. Therefore, incorporating TEG when managing cirrhotic patients with nonvariceal upper GI bleeding appears to be beneficial as it limits the need for transfusion of limited resources.
Author’s Conclusion: “Among patients with advanced cirrhosis with coagulopathy and nonvariceal upper GI bleeding, TEG guided transfusion strategy leads to a significant lower use of blood components compared with SOC (transfusion guided by INR and PLT count), without an increase in failure to control bleed, failure to prevent rebleed, and mortality.”
Our Conclusion
Overall, we agree with the authors’ findings. Patients with advanced cirrhosis and nonvariceal upper GI bleeding transfused using a TEG-guided strategy required less FFP, platelets, and cryoprecipitate. In addition, they experienced fewer transfusion-related reactions, with no difference in mortality rates compared to the SOC group. We would advocate for using a TEG-guided transfusion strategy in this patient population as it would conserve precious resources while obtaining similar health outcomes. However, EGD results confirming nonvariceal bleeding would likely be unavailable outside the ICU.
References
- De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology. 2016;63(2):566-573. PMID: 26340411
- Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products – A systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25(1):39. Published 2017 Apr 13. PMID: 28403868
- Hayes-Larson E, Kezios KL, Mooney SJ, Lovasi G. Who is in this study, anyway? Guidelines for a useful Table 1. J Clin Epidemiol. 2019;114:125-132. PMID: 31229583
- Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography-Guided Blood Component Use in Patients With Cirrhosis With Nonvariceal Bleeding: A Randomized Controlled Trial. Hepatology. 2020;71(1):235-246. PMID: 31148204
- Rout G, Shalimar, Gunjan D, et al. Thromboelastography-guided Blood Product Transfusion in Cirrhosis Patients With Variceal Bleeding: A Randomized Controlled Trial. J Clin Gastroenterol. 2020;54(3):255-262. PMID: 31008867
- Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726-732. PMID: 20335313
- Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: a randomized clinical trial. Transplant Proc. 2010;42(7):2590-2593. PMID: 20832550
For More Thoughts on This Topic
Guest Post By:

Cheng Ng, MD
PGY-1, Emergency Medicine Resident
Nuvance Health, Poughkeepsie, New york
Email: cheng.ng@nuvancehealth.org

Marco Propersi, DO FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)