 Background: In 2011, we saw 7 million patients in the emergency department (ED) complaining of chest pain. Most of these patients did NOT have an acute coronary syndrome (ACS) or an acute myocardial infarction (AMI). Missing an AMI is one of the biggest fears we have in the ED. By using validated risk scores, we can help decrease the risk of missing AMI and the resultant adverse events. There are multiple scores available for our use. Thrombolysis in Myocardial Infarction (TIMI) predicts risk of adverse outcomes in the next 14 days. Global Registry of Acute Coronary Events (GRACE) predicts outcomes at 6 months. ED specific scores include HEART and Emergency Department Assessment of Chest Pain (EDACS). But, how well do these scores actually perform? Are we missing AMIs by using these clinical risk scores?
Background: In 2011, we saw 7 million patients in the emergency department (ED) complaining of chest pain. Most of these patients did NOT have an acute coronary syndrome (ACS) or an acute myocardial infarction (AMI). Missing an AMI is one of the biggest fears we have in the ED. By using validated risk scores, we can help decrease the risk of missing AMI and the resultant adverse events. There are multiple scores available for our use. Thrombolysis in Myocardial Infarction (TIMI) predicts risk of adverse outcomes in the next 14 days. Global Registry of Acute Coronary Events (GRACE) predicts outcomes at 6 months. ED specific scores include HEART and Emergency Department Assessment of Chest Pain (EDACS). But, how well do these scores actually perform? Are we missing AMIs by using these clinical risk scores?
What They Did:
- This study was part of a larger, multicenter, prospective observational study. The original study was looking to validate a novel cardiac troponin for ED use in patients suggestive of ACS.
- 7 geographically different academic US EDs
- Inclusion: patients age 21 or older with symptoms suggestive of ACS
- Exclusion: implanted defibrillator discharge < 24 hours, cardiac procedure or bypass < 30 days, recent chest trauma
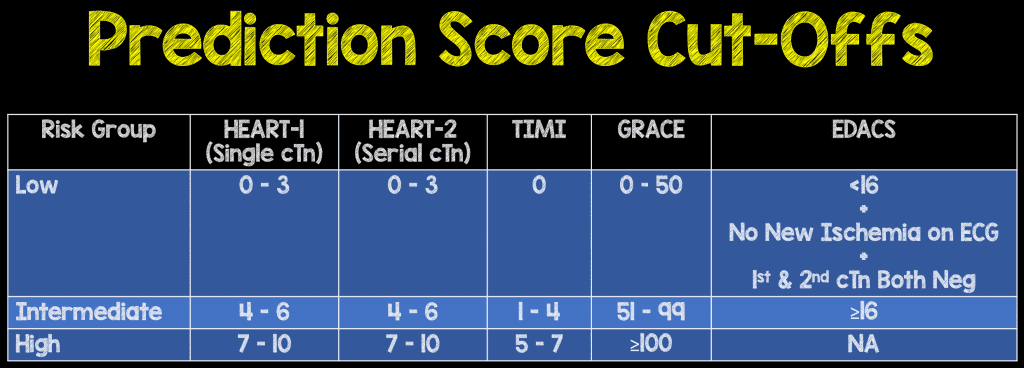
- Recorded: clinical gestalt, TIMI, GRACE, HEART-1, HEART-2, and EDACS to separate into low, intermediate, and high risk. EDACS only uses low and not low risk classifications. (Difference between HEART-1 and HEART-2 was using one or two troponins.)
- Cutoffs used: HEART 0-3, TIMI = 0, GRACE < 50, EDACS < 16
Outcomes:
- Primary—presence or absence of AMI. Final diagnosis was determined by a committee of 3 (2 cardiologists, 1 ED), blinded to each other’s determination. The ESC/ACCF/AHA/WHR 3rd Universal definition of MI was used.
Results:
- 434 patients included in the final analysis
- Median Age: 57 years (Range: 49 – 64)
- Diagnosis of AMI: 18.4%
- Median Time Between Serial cTn: 4.1 hr (Range 2.7 – 7.8hr)
Strengths:
- Adequate enrollment: Study needed at least 45 patients with AMI and assumed a 10% prevalence for AMI. They would need 450 patients enrolled in the study (They enrolled 459 of which 434 were included in the final analysis).
- Use of all major risk assessment scores at recommended cutoffs
Limitations:
- Only 82 patients had confirmed AMI: This is a small sample size
- Possible selection bias, excluded many patients due to incomplete data: This could lead to under- or over-estimation of clinical risk scores
- Only used AMI, likely missed other ACS and longer-term major adverse cardiac events (MACE)
- Each hospital troponin was different, with variable cutoffs. This may have caused variability in the results of the prediction scores this study
- Half of the false negative cases involved point of care assays. The high rate of missed AMI in patients classified as low risk may be due to using older, less sensitive cTn assays, which have high coefficients of variation
- Some facilities used POC cTnI as their initial test and laboratory cTnI as their follow up test, which makes it difficult to evaluate rise and fall as two different assays were used
Discussion:
- Of all the symptoms described diaphoresis was much more common in patients diagnosed with an AMI vs patients without AMI (43.8% vs 5.4%). Take diaphoresis seriously.
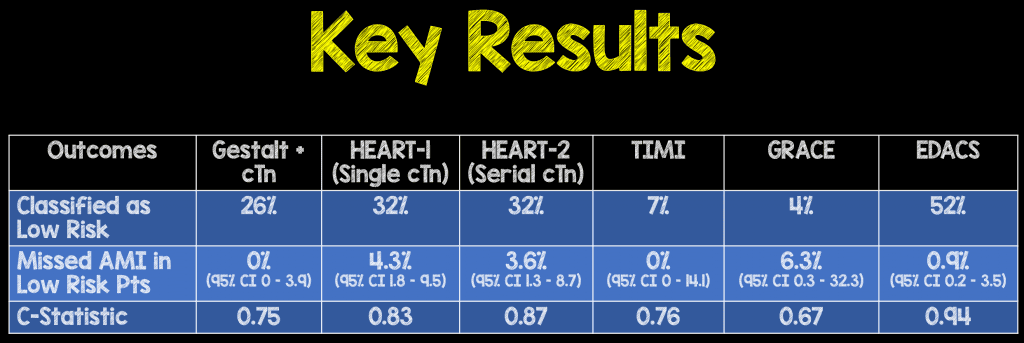
- All assessments were within statistical variability, but high miss rates were noted in all except TIMI, and gestalt + troponin.
- Reducing score cutoffs would result in zero misses, but much fewer patients would be classified as low risk. The lower cut-offs were defined as:
- HEART-1 = 1
- GRACE </= 48
- TIMI= 0
- HEART-2 </= 2
- EDACS </= 11
- The percentage of patients that would be considered low risk with these lower cut offs come at the price of less patients identified as low risk:
- HEART-1: 1%
- GRACE: 3.3%
- TIMI: 7%
- HEART-2: 19.5%
- EDACS: 33.6%
- It is important to note that the original HEART score and EDACS score have had prospective evaluation. Most of the HEART score studies are performed in European populations and the EDACS score was derived and validated in New Zealand but used hs cTnI. The findings of this study may simply be due to different populations (European vs US) or the use of hs cTnI (which until recently was not available in the US). Even so, due to the smaller population of this study, 95% CIs were very wide.
- Just a little discussion point about risk scores. Risk scores are tools to help clinicians have a standardized approach to patient complaints. There will always be exceptions to the tools. Not to pick on anyone one score, but lets talk about the HEART-2 score as an example. It is possible that someone could have a low risk score (0 – 3) and not truly be low risk.
- Lets start with risk factors. If a patient doesn’t go to see a doctor, then they won’t have any past medical history, when in fact they may. This will grossly underestimate their risk.
- Although only one ECG is required for the HEART-2 score, there is something to be said for multiple ECGs. Be sure to look at pre-hospital ECGs, old ECGs in your EMR, and for god’s sake, get serial ECGs in patients you are concerned about. Remember, a single ECG is one point in time. We don’t want to miss dynamic changes and the best way to find these subtle changes may be looking at pre-hospital ECGs, old ECGs, and serial ECGs.
- What about troponins? It is very possible that you could have a patient that doesn’t go to doctors, and therefore has no risk factors, a normal ECG and have a positive troponin. By definition, this would still be low risk if you use the HEART-2 score at face value. I think most of us would agree that positive troponins are not low risk. Even if it is not ACS that is causing the elevation, there maybe another pathology at play. This is not a low risk patient, and should not be sent home without further risk stratification or workup.
Author Conclusion: “Using their recommended cutpoints and non-high sensitivity cTn, TIMI and unstructured clinical impression were the only scores with no missed cases of AMI. Using lower cutpoints (GRACE ≤48, TIMI = 0, EDACS ≤11, HEART ≤2) missed no case of AMI, but classified less patients as low-risk.”
Clinical Take Home Point: Clinical gestalt remains the most useful tool for assessment of risk of ACS/AMI. The use of risk assessment tools should be regarded as just that, tools. Keep in mind the limitations of each as you are taking care of patients.
References:
- Singer AJ et al. Missed Myocardial Infarctions in ED Patients Prospectively Categorized as Low Risk by Established Risk Scores. Am J Emerg Med 2017. [Epub Ahead of Print] PMID: 28108220
For More on This Topic Checkout:
- Ryan Radecki at EMLit of Note: Use HEART, or Whatever
Post Peer Reviewed By: Salim Rezaie (Twitter: @srrezaie)



