To date, there is no high-quality evidence to support the use of therapeutic anticoagulation in hospitalized critically ill patients with COVID-19. A recent multiplatform trial [Link is Here] and the HEP-COVID Trial [Link is here] found clinical improvement with therapeutic anticoagulation in non-ICU patients hospitalized with COVID-19. However, previous trials used treatment strategies utilizing heparin and enoxaparin. The ACTION Trial is the first (and currently the only) trial to investigate therapeutic anticoagulation with direct oral anticoagulants (DOACs) for the management of hospitalized patients with COVID-19.
Article: Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. [PMID: 34097856]
Clinical Question: Does in-hospital therapeutic anticoagulation in patients with COVID-19 decrease the time to death, duration of hospitalization, or duration of supplemental oxygen, when compared to prophylactic anticoagulation?
What They Did:
- Pragmatic, open-label, multicenter, randomized controlled trial in patients hospitalized with COVID-19 with elevated D-dimer.
- Patients were randomly assigned to receive
- Therapeutic anticoagulation for 30 days
- Stable patients received rivaroxaban
- Unstable received enoxaparin or heparin
- OR prophylactic anticoagulation with enoxaparin or heparin
- Therapeutic anticoagulation for 30 days
- Randomized in 1:1 permuted blocks
Population:
Inclusion:
- Confirmed diagnosis of COVID-19 admitted to hospital (RT-PCR, antigen test or IgM test)
- Duration of symptoms ≤14 days
- Patients ≥ 18 years old
- D-dimer concentration above the upper limit of normal
Exclusion:
- Patients with a specific indication for therapeutic anticoagulation (e.g, diagnosis of VTE, AF, mechanical valve prosthesis)
- Platelets <50,000 /mm3
- Use of aspirin >100 mg
- Use of P2Y12 inhibitor (clopidogrel, prasugrel, ticagrelor)
- Chronic use of NSAIDs
- Sustained uncontrolled systolic BP ≥180 mm Hg or diastolic BP ≥100 mm Hg
- INR >1.5
- Contraindication to therapeutic anticoagulation
- Patients with DIC
- Any intracranial bleeding at any time in the past or current intracranial neoplasm, cerebral metastases, arteriovenous malformation, or aneurysm
- Active cancer (excluding non-melanoma skin cancer) is defined as cancer, not in remission or requiring active chemotherapy or adjunctive therapies such as immunotherapy or radiotherapy
- Hypersensitivity to rivaroxaban
- Use of strong inhibitors of cytochrome P450 (CYP) 3A4 and/or P-gp (e.g., protease inhibitors, ketoconazole, itraconazole) and/or use of P-gp and strong CYP3A4 inducers (including, but not limited to, rifampin/rifampicin, rifabutin, rifapentine, phenytoin, phenobarbital, carbamazepine, or St. John’s Wort)
- Known HIV infection
- Creatinine clearance <30 mL/min
- Pregnancy or breastfeeding
Intervention:
- Stable patients received oral rivaroxaban 20 mg once daily adjusted for renal clearance
- Unstable patients received enoxaparin OR unfractionated heparin
- Transitioned to rivaroxaban when stable
Control:
- Prophylactic anticoagulation with enoxaparin or unfractionated heparin
Outcomes:
Primary Outcome:
- Hierarchical Composite through 30 days:
- Time to death
- Duration of hospitalization
- Duration of supplemental oxygen
Secondary Outcomes:
- Two composite outcomes with and without all-cause mortality and:
- Venous Thromboembolism (VTE)
- Acute myocardial infarction (MI)
- Stroke
- Systemic Embolism
- Major adverse limb event
- Duration of Supplemental oxygen or non-invasive or mechanical ventilation
- Disease progression
- Mild disease = no criteria to be classified as “moderate” or “severe”
- Moderate disease = oxygen saturation <94% or pulmonary infiltrates >50% or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300.
- Severe disease = respiratory failure or hemodynamic instability or multiple organ dysfunction.
- Rehospitalization
- WHO 8-point ordinal scale:
- Death
- Invasive mechanical ventilation + support for another organ dysfunction
- Invasive mechanical ventilation alone
- Noninvasive ventilation/high-flow oxygen
- Hospitalized on supplemental oxygen
- Hospitalized not requiring supplemental oxygen
- Not hospitalized, limitation on activities and/or requiring home oxygen;
- Not hospitalized, no limitations on activities.
Primary Safety Outcome:
- Major bleeding or clinically relevant non-major bleeding
- Defined according to International Society in Thrombosis and Hemostasis (ISTH) Criteria
Net Benefit Outcome:
- Composite of primary efficacy outcome and the primary safety outcomes
Results:
- 3331 patients screened
- 615 patients enrolled
- 311 patients randomized to receive therapeutic anticoagulation
- 304 patients randomized to receive prophylactic anticoagulation
- > 99% of patients received anticoagulation according to the study protocol
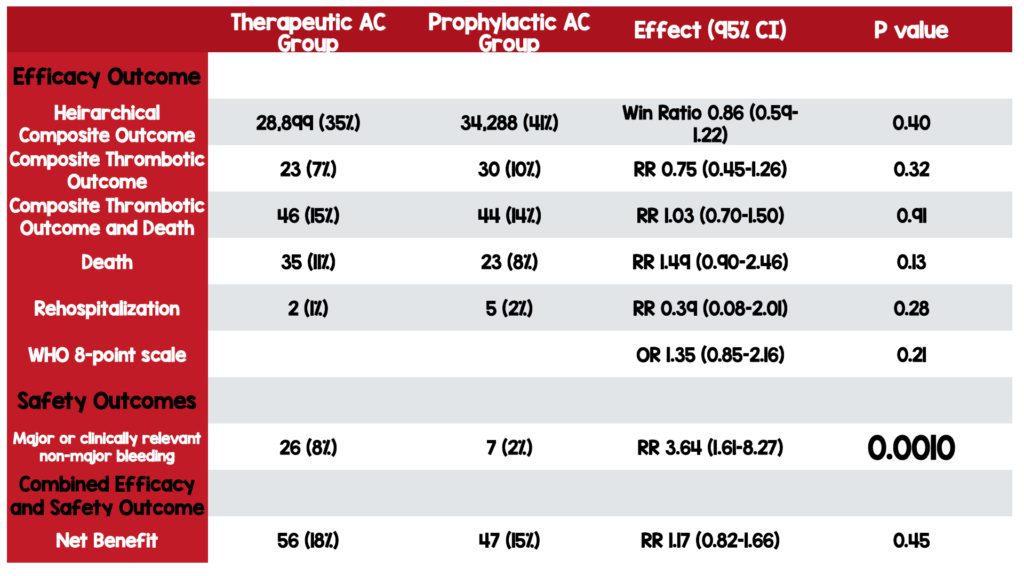
Primary Outcome Results:
- NO STATISTICALLY SIGNIFICANT DIFFERENCE BETWEEN GROUPS
- 28,899 (34.8%) wins in the therapeutic group
- 34,288 (41.3%) wins in the prophylactic group
- 19,837 (23.9% ties
- Win Ratio 0.86 [95% CI 0.59–1.22] p=0.40
- Analysis of individual components of the primary outcome showed no difference between groups.
- NO DIFFERENCE in time to death
- NO DIFFERENCE in the duration of hospitalization
- NO DIFFERENCE in supplemental oxygen
Secondary Outcome Results:
- NO STATISTICALLY SIGNIFICANT DIFFERENCE BETWEEN GROUPS
- The 8-point ordinal scale
- Disease progression
- Duration of invasive mechanical ventilation
- Incidence of individual thrombotic events
- Composite of VTE, MI, stroke, systemic embolism, or major adverse limb events
Primary Safety Outcome Results:
- Major or clinically relevant bleeding
- STATISTICALLY SIGNIFICANT DIFFERENCE FOUND BETWEEN GROUPS
- 26 (8%) patients receiving therapeutic anticoagulation
- 7 (2%) patients receiving prophylactic anticoagulation
- RR 3.64 [95% CI 1.61–8.27] p= 0.0010
- Any Bleeding
- STATISTICALLY SIGNIFICANT DIFFERENCE FOUND BETWEEN GROUPS
- 36 (12%) patients receiving therapeutic anticoagulation
- 9 (3%) patients receiving prophylactic anticoagulation
- RR 3.92 [95% CI 1.92–8.00] p< 0.0001
Net Benefit Outcome Results:
- NO STATISTICALLY SIGNIFICANT DIFFERENCE BETWEEN GROUPS
- RR 1.17 [95% CI 0.82–1.66] p= 0.45
Strengths:
- Asks an impactful and timely patient-centered question
- Outcomes were patient-oriented
- Multicenter randomized control study design increases the generalizability
- Blind adjudication of outcomes limits bias in an open-label trial
- Performed an intention to treat analysis
- Analyzed participants in groups in which they were randomized regardless of adherence to study protocol
- More reflective of a real-world scenario
- Adherence to study protocol was excellent and > 99% of participants received anticoagulation according to the study protocol.
- Compliance with outpatient therapy was also good
- 94.8% in therapeutic group
- 99.5% in the prophylactic group
Limitations:
- The screened population appears to be a convenience sample.
- It’s unclear how many patients were eligible for enrollment.
- 3331 patients screened at 31 sites in 8 months during a pandemic
- 13 patients screened per month at each site, OR just under 2.5 patients enrolled per month from each site
- During a surge in Brazil, we expect hundreds of patients were likely hospitalized daily across 31 sites.
- The open-label study design increases bias.
- This study was performed in a single country limiting generalizability.
- The primary outcome was a composite
- Not all components of the composite are equal
- Included patients with a positive COVID-19 test within14 days
- The average time from symptom onset to randomization was 10 days
- Its possible anticoagulation may only be beneficial early in the course of disease before the advent of a hyperinflammatory state and cytokine storm.
- Investigated a treatment strategy and not a specific therapy.
- The protocol allowed for change to different therapies within each study arm.
- The protocol did not require diagnostic imaging to detect thrombotic complications.
Discussion:
- The Win Ratio
- Win ratio prioritizes more clinically relevant outcomes (e.g. death).
- All patients in the intervention group are compared to the control group within each stratum (stable vs. unstable).
- Participants were first compared for time until death, then the length of hospital stay, and finally the length of oxygen supplementation.
- The participant who lives longer is the “winner.” If tied, participants are next compared in the length of hospital stay. In the event of another tie, participants are then compared in the length of oxygen supplementation.
- If all three components are the same, a “tie” is entered as the final result.
- Win ratio: the number of wins is divided by the total number of losses. A value > 1 indicates a better outcome for the therapeutic anticoagulation group.
- The investigators considered a difference of at least 1 day later for “time until death,” and 2 days later for “length of hospital stay,” and length of oxygen supplementation.”
- Therefore, 1 day on hi-flow is considered the same as 3 days.
- In a pandemic, a difference in 2 days’ utilization of medical equipment and resources may be critically important.
- Inside the Numbers
- There was no statistically significant difference in any primary or secondary outcome
- Subgroup analysis showed similar results with no differences in groups
- Primary safety outcome achieved statistical significance
- In this trial, For every 17 patients treated with therapeutic anticoagulation, 1 patient would suffer major or clinically relevant bleeding.
- Investigation of a Treatment Strategy
- Clinically unstable patients received enoxaparin or heparin instead of rivaroxaban.
- The study protocol allowed for change between different drugs as long as the drugs were within the study group.
- The use of multiple therapies in either study arm could mask the potential benefits or harms of rivaroxaban.
- Composite Primary Endpoints
- Investigators are essentially studying the degree to which anticoagulation prevents thrombotic complications that contribute to morbidity and mortality.
- However, they did not require diagnostic imaging which introduces subjectivity and bias.
- The time until death, length of hospitalization and length of oxygen supplementation are poor measures for thrombotic events.
- The use of hierarchical endpoints with win ratio analysis somewhat mitigates the limitations of traditional composite outcomes.
- However, there is little doubt patients will care less about how long they took to die versus if they died.
Current Evidence Regarding Anticoagulation in COVID-19
- This trial contradicts the findings of other recent publications in a similar cohort that showed a benefit of therapeutic anticoagulation.
- Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. [PMID: 34351721]
- Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. [PMID: 34617959]
- Important Differences:
- HEP–COVID trials included patients <72 hours from positive COVID-19 test
- The Multiplatform Trial included patients enrolled from 3 separate investigators.
- ATTACC and ACTIV-4a investigators included patients < 72 hours from hospitalization or COVID-19 confirmation
- Represents 1938 of 2231 patients in the trial
- REMAP-CAP included patients <14 days since hospitalization
- The HEP–COVID Trial used a D-dimer cutoff 4x the upper limit of normal.
- Trials separated ICU and non-ICU patients
- Trials evaluated the use of parenteral anticoagulants, not DOACs
- The findings are similar to other recent studies that showed no improvement in clinical outcomes with therapeutic anticoagulation.
- The INSPIRATION Randomized Clinical Trial [PMID: 33734299]
- Therapeutic anticoagulation with heparin in critically ill patients with COVID-19 [PMID: 34351722]
- Heparin for Moderately Ill Patients with Covid-19 [PMID: 34268513]
Authors Conclusions: “In patients hospitalised with COVID-19 with elevated D-dimer concentration, initial in-hospital therapeutic anticoagulation with rivaroxaban for stable patients or enoxaparin for unstable patients followed by rivaroxaban through 30 days did not improve clinical outcomes and increased bleeding compared with in-hospital prophylactic anticoagulation. Thus, the use of therapeutic-dose rivaroxaban, and other direct oral anticoagulants, should be avoided in hospitalised patients with COVID-19 who do not have an evidence-based indication for oral anticoagulation.”
Our Conclusions: In this trial, therapeutic dose rivaroxaban did not improve clinical outcomes and increased bleeding compared to a prophylactic dose anticoagulation strategy with enoxaparin or heparin. However, other recent trials may have captured a select cohort of patients earlier in the course of disease (<72 hours) and who may be at higher risk for thrombotic events (D-dimer 4x upper limit of normal) that may benefit from therapeutic dose anticoagulation with parenteral medicines.
Clinical Bottom Line:
At this point, there is no evidence for the use of DOACs for therapeutic anticoagulation for preventing thrombotic events in hospitalized patients with COVID-19.
For More on This Topic Checkout:
- REBEL EM: The MICHELLE Trial: Anticoagulation Post-Discharge in Patients Hospitalized Secondary to COVID-19
- REBEL EM: The ACTIV-4b Trial: Antithrombotics For Treatment of Outpatient COVID-19
- REBEL EM: The HEP-COVID Trial: Therapeutic Anticoagulation in Non-Critically Ill COVID-19 Patients
- REBEL EM: COVID-19 and Anticoagulation: Full Dose or Prophylactic Dose?
- Critical Care Reviews: mpRCT Anticoagulation Trial
- St. Emlyn’s Blog: Thromboprophylaxis for the Non ICU Hospitalised COVID-19 Patient
References:
- Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. [PMID: 34097856]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):790-802. [PMID: 34351721]
- Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial [published correction appears in JAMA Intern Med. 2022 Feb 1;182(2):239]. JAMA Intern Med. 2021;181(12):1612-1620. [PMID: 34617959]
- INSPIRATION Investigators, Sadeghipour P, Talasaz AH, et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620-1630. [PMID: 33734299]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):777-789. [PMID: 34351722]
- Sholzberg M, Tang GH, Rahhal H, et al. Heparin for Moderately Ill Patients with Covid-19. Preprint. medRxiv. 2021;2021.07.08.21259351. Published 2021 Jul 12. [PMID: 34268513]
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)



