Paper: Beigel JH et al. Remdesivir for the Treatment of COVID-19 – Preliminary Report. NEJM 2020. [Epub Ahead of Print]
Clinical Question: Does remdesivir reduce time to recovery in hospitalized patients with COVID-19 when compared to placebo?
What They Did:
- This is the 1st part of a series of multicenter, phase 3, double-blind, placebo-controlled trials of IV remdesivir vs placebo in adults hospitalized with COVID-19
- 60 trial sites and 13 subsites globally
- Remdesivir = 200mg loading dose day 1, followed by 100mg qD up to 9 additional days
- Placebo = 10 days
Outcomes:
- Primary: Time to recovery (Defined as the 1st day during the 28d after enrollment on which the patient was category 1, 2, or 3 based on the ordinal scale below)
- Eight Category Ordinal Scale:
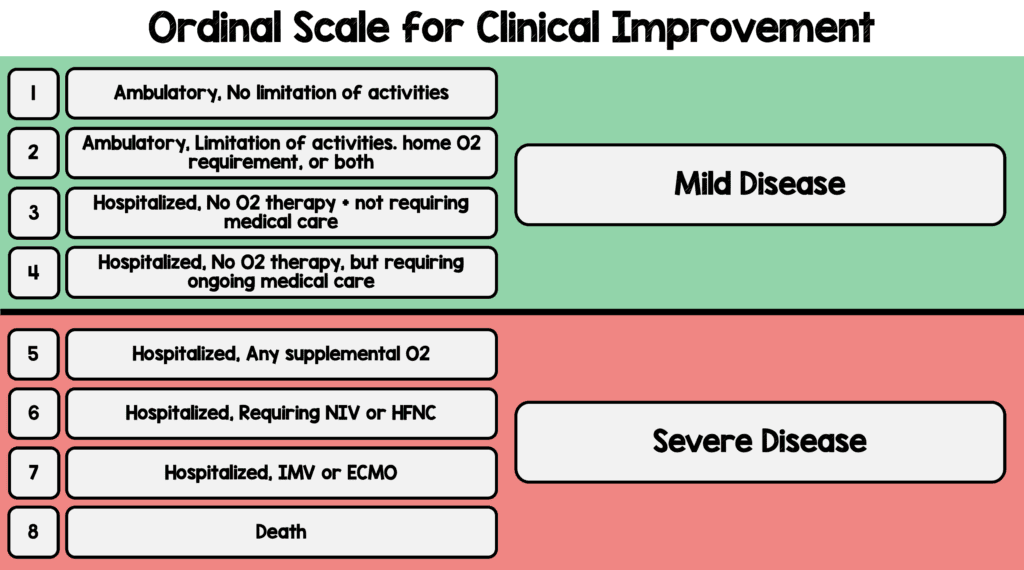
- Secondary:
- 14 and 28d mortality
- Grade 3 and 4 adverse events and serious adverse events
- There were a slew of other secondary outcomes including change from baseline of multiple labs (ALT, AST, creatinine, glucose, hemoglobin, platelets, PT, total bilirubin, and WBC with differential)
- The clinicaltrials.gov website lists the rest of the secondary outcomes (It is a ridiculously long list) [Link is HERE]
Inclusion:
- Age ≥18 years
- Hospitalized with symptoms suggestive of COVID-19
- Symptoms suggestive of lower respiratory tract infection:
- Radiographic infiltrates by imaging study
- Peripheral O2 sat ≤94% on RA
- Requiring supplemental 02, mechanical ventilation, or ECMO
Exclusion:
- ALT/AST >5x ULN
- Impaired renal function or need for hemodialysis or hemofiltration (the cutoff of what impaired renal function means is not specifically stated in the manuscript)
- Allergy to study product
- Pregnancy or breast feeding
- Anticipated discharge from the hospital or transfer to another hospital within 72hrs of enrollment
Results:
- 1063 patients underwent randomization
- 1059 are included in the analysis
- Remdesivir: 538pts
- Placebo: 521pts
- Median number of days between symptom onset and randomization was 9d (Range 6 to 12d)
- 943 patients (88.7% had severe disease at enrollment: Mild/moderate disease was defined by a SpO2>94% and respiratory rate <24BPM without supplemental oxygen)
- Would argue that category 5 (Hospitalized, requiring any supplemental oxygen) is moderate disease and not severe disease. Therefore, looking at Category 6 and 7 as severe disease, this number should be more like 44.1%)
- Median Recovery Time (Primary Outcome):
- Remdesivir: 11d (95% CI 9 to 12)
- Placebo: 15d (95% CI 13 to 19)
- Rate Ratio for recovery: 1.32; 95% CI 1.12 to 1.55; p<0.001
- 14d Mortality:
- Remdesivir: 32/538 (5.9%)
- Placebo: 54/521 (10.4%)
- HR 0.70; 95% CI 0.47 to 1.04 (Not statistically significant)
- Serious Adverse Events:
- Remdesivir: 21.1%
- Placebo: 27.0%
- No deaths were considered to be related to treatment assignment
ADDENDUM (10/10/2020): Final Report of ACTT-1 Released in NEJM
Results:
- 1062 patients randomized
- Median Recovery Time:
- Remdesivir: 10d
- Placebo: 15d
- Rate ratio for recovery 1.29; 95% CI 1.12 to 1.49; p<0.001
- Median Recovery Time in Severe Disease:
- Remdesivir: 11d
- Placebo: 18d
- Rate ratio for recovery 1.31; 95% CI 1.12 to 1.52
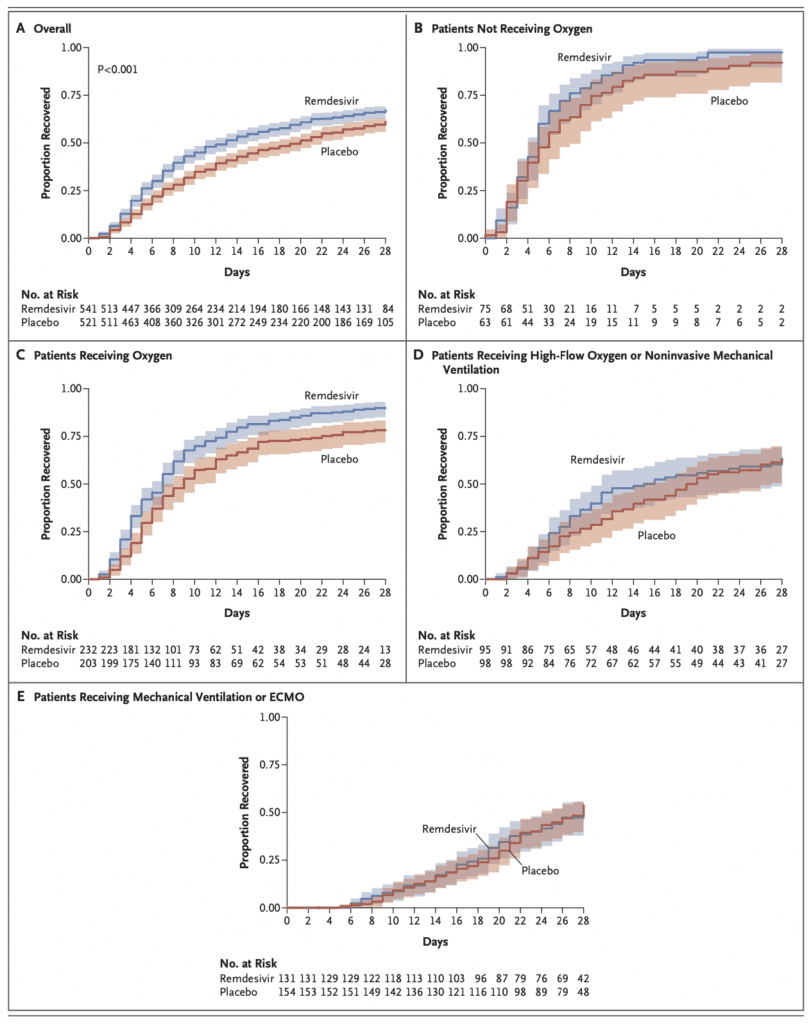
- Looking at the Kaplan-Meier Estimates of recovery, the group that had the most benefit from remdesivir was patients on low flow O2, BUT not patients on HFNC, NIV, IMV, or ECMO (This was also seen in secondary outcomes)
- 15d Mortality:
- Remdesivir: 6.7%
- Placebo 11.9%
- HR 0.55; 95% CI 0.36 to 0.83
- 29d Mortality:
- Remdesivir: 11.4%
- Placebo: 15.2%
- HR 0.73; 95% CI 0.52 to 1.03 (Not Statistically Significant)
- Serious Adverse Events:
- Remdesivir: 24.6%
- Placebo 31.6%
Strengths:
- Multicenter, international double-blind, placebo-controlled trial (All the words we love to see when evaluating a trial)
- >98% of patients received the medication they were randomized to in both arms
- No imbalances in baseline characteristics of patients between groups
Limitations:
- Changed the primary outcome of the study
- Although off-label medications specific to treatment of COVID-19 were prohibited from day 1 to 29 of this study, these medications could have been used before enrollment into the trial
- Due to the early stoppage, treating physicians could request to be made aware of the treatment assignment of patients who had not completed their medication regimen if clinically indicated (i.e. worsening clinical status), and patients originally in the placebo group could be given remdesivir (i.e. early unblinding).
- There was also unblinding of some patients as they didn’t actually have placebo to give patients. The authors do not discuss how many patients were unblinded from the start of treatment
- Early unblinding could unfortunately overexaggerate effect size of remdesivir
- 65 – 70% of patients in both arms had completed the study at the time of this publication. Not everyone in the study had completed the full 29d course, meaning we still don’t have outcomes for these patients. This could impact the final results.
- 28-day Kaplan-Meier estimates of mortality not reported in this manuscript given large number of patients had not yet completed 29 day visits
- Lots of data missing from this report (i.e. baseline labs, vitals, duration of oxygen therapy, etc…)
Discussion:
- Data and safety monitoring committee recommended early unblinding of the results on the basis of the findings from the interim analysis that showed shortened time to recovery in the remdesivir group compared to placebo and provided the results to the NIAID and results were made subsequently public
- Enrollment was already completed at the time of this early stoppage, but only 482 recoveries and 81 deaths had been entered into the database
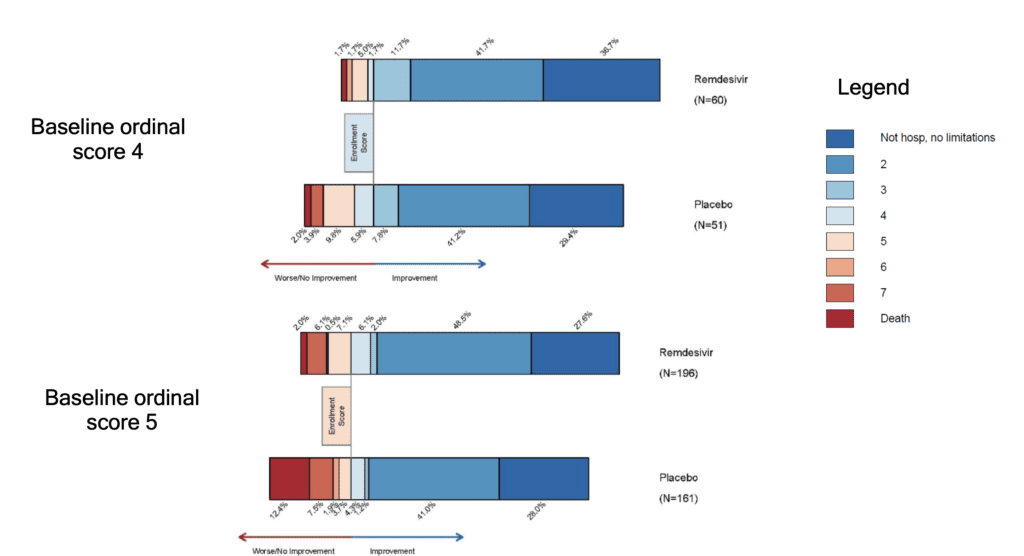
- The benefit of remdesivir was most apparent in patients with a baseline ordinal score of 5 (requiring oxygen). This could simply be due to a larger sample size in this category since the interaction test of treatment by baseline score on the ordinal scale was not significant
- The primary outcome of the study was changed, however authors state investigators and statisticians remained blinded (only 72 patients had been enrolled at the time of change)
- At the end of the discussion, the authors state, “However, given the high mortality despite the use of remdesivir, it is clear that treatment with an antiviral drug alone is not likely to be sufficient. Future strategies should evaluate antiviral agents in combination with other therapeutic approaches or combinations of antiviral agents to continue to improve patient outcomes in COVID-19.”
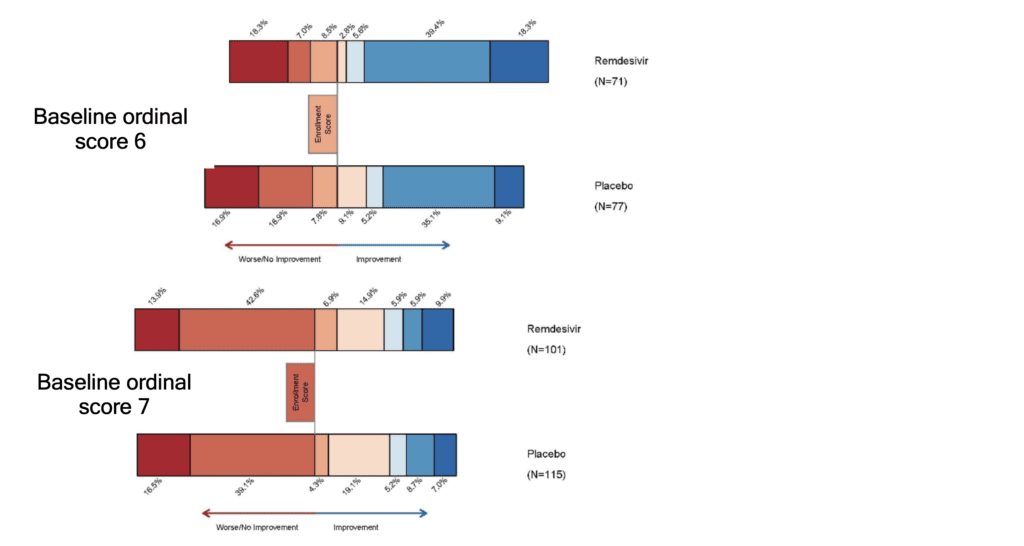
- In the supplement figure S1, is a 15-day outcome by baseline ordinal scale in the intention to treat population. This figure shows that there is a more impressive decrease in progression of disease in patients with lesser severity of illness, but no real improvements in patients who require NIV/HFNC or mechanically ventilated/on ECMO (Baseline ordinal scores of 6 and 7).
Author Conclusion: “Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 and evidence of lower respiratory tract infection.”
Clinical Take Home Point:
- Part 1 of the ACTT trial is far from perfect
- Although there was significant unblinding and a change in the primary outcome, the upside of this trial is we see a 4 day decrease in in days to clinical improvement (11d vs 15d) favoring remdesivir (Although not in patients requiring HFNC/NIV/IMV/ECMO)
- Remdesivir numerically decreased 14d mortality, but this finding was not statistically significant. We will have to see the results of the patients who have yet to complete the study to see if this trend stands or not
- In the middle of a pandemic, in hospitals getting a surge of patients, a 4 day decrease in recovery time can have significant ramifications on a health system
References:
- Beigel JH et al. Remdesivir for the Treatment of COVID-19 – Preliminary Report. NEJM 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- REBEL EM: COVID-19 – Two More Trials Just Published on Remdesivir
- PulmCrit: ACTT-1 Preliminary Report on Remdesivir
- EMNerd: The Case of the Partial Cohort
- The Bottom Line: ACTT-1
- First10EM: Remdesivir – The ACTT-1 Trial
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)





