As of May 2021, approximately 5 million people (≈55% of the population) in Israel, had received two doses of the BNT162b2 (Pfizer) vaccine. We now have a large data set from one of the largest health care organizations in Israel to compare the incidence of potential adverse events among vaccinated persons vs matched unvaccinated persons as well as incidence of adverse events associated with SARS-CoV-2 infection (the disease that these interventions aim to prevent or treat).
Paper: Barda, N et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. NEJM 2021. PMID: 34432976
Clinical Question: What is the risk of adverse outcomes after BNT162b2 mRNA COVID-19 vaccination compared to natural infection with SARS-CoV-2?
What They Did:
- Prospective observational case matched trial
- Case matched vaccinated persons to unvaccinated persons according to sociodemographic and clinical variables
- Similarly, also case matched SARS-CoV-2 infected persons to uninfected persons
- Risk ratios and risk differences at 42 days after vaccination were derived
Outcomes:
- Incidence of adverse events up to 42 days after vaccination or infection
Inclusion:
- ≥16 years of age
- Continuous membership in the health care organization for a full year
- No previous SARS-CoV-2 infection
- No contact with the health care system in the previous 7 days (This criterion was included as an indicator of a health event not related to subsequent vaccination that could reduce probability of receiving the vaccine)
Exclusion:
- Populations where confounding could not be adequately addressed
- Long-term care facility residents
- Confined to homes for medical reasons
- Health care workers
- Data on body-mass index or residential area were missing
Results:
- 884,828 thousand people in each group (Vaccinated vs Unvaccinated)
- 4% of eligible persons were successfully matched
- Cohort median age = 38 years
- Female sex = 48%
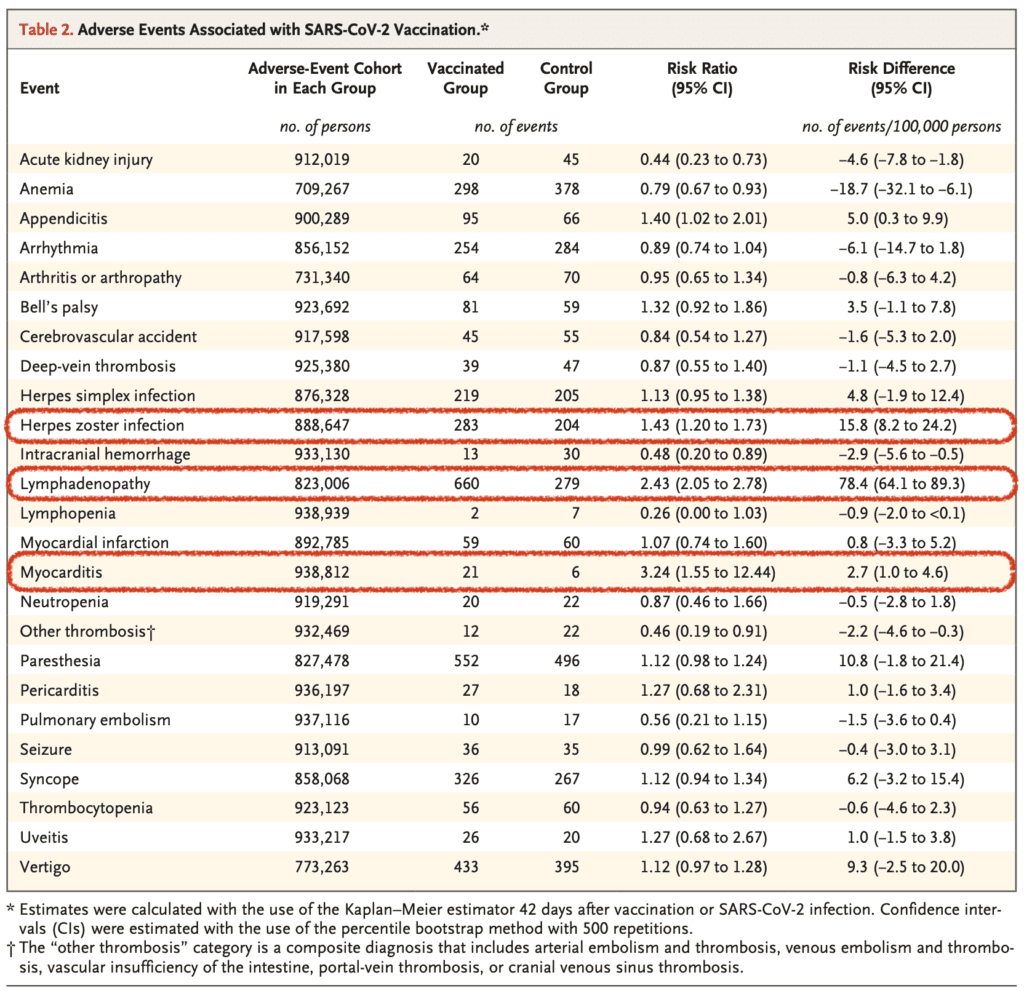
- The 3 most common adverse effects seen in the vaccinated vs unvaccinated populations:
- Myocarditis: Incidence was higher after 2nd vaccination
- Lymphadenopathy
- Appendicitis
- Herpes Zoster Infection
- Vaccination compared to no vaccination was more protective against:
- Anemia
- Acute Kidney Injury
- Intracranial Hemorrhage
- Lymphopenia
- 173,106 people in each group (Infection vs No Infection)
- 8% of the eligible persons were successfully matched
- Cohort median age = 34 years
- Female sex = 54%
- Comparison of Vaccinated vs Unvaccinated to Infection vs No Infection
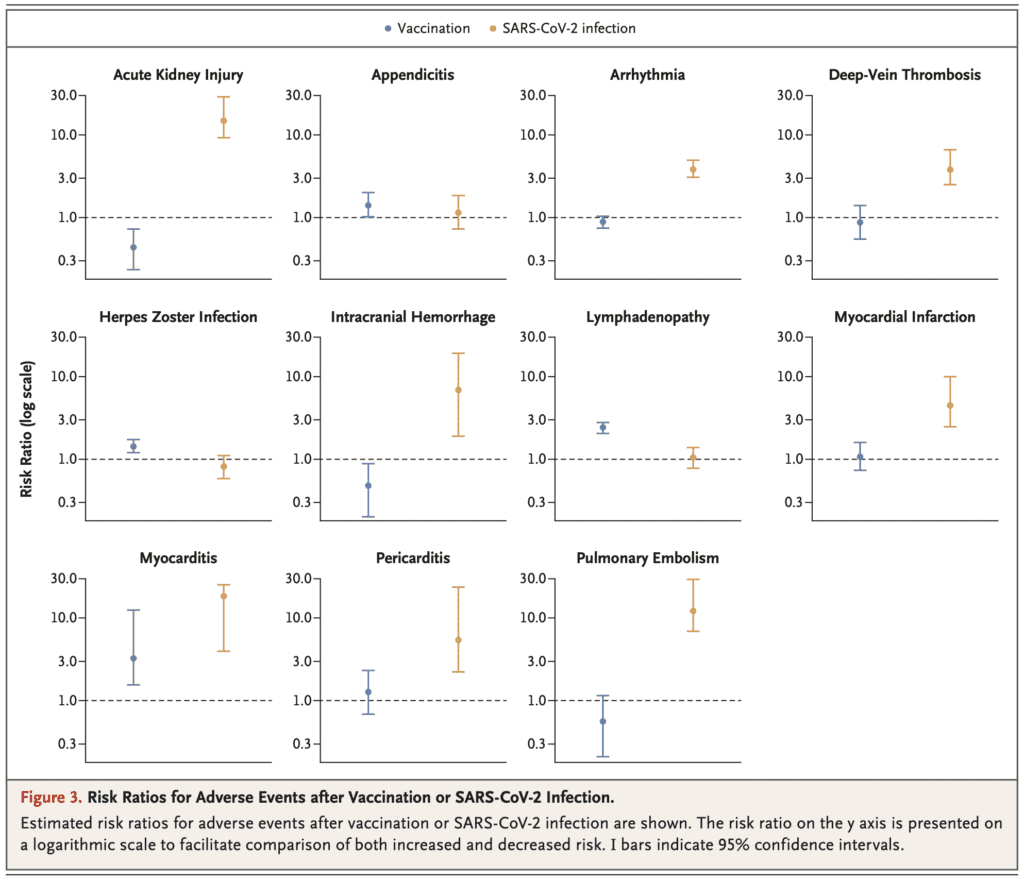
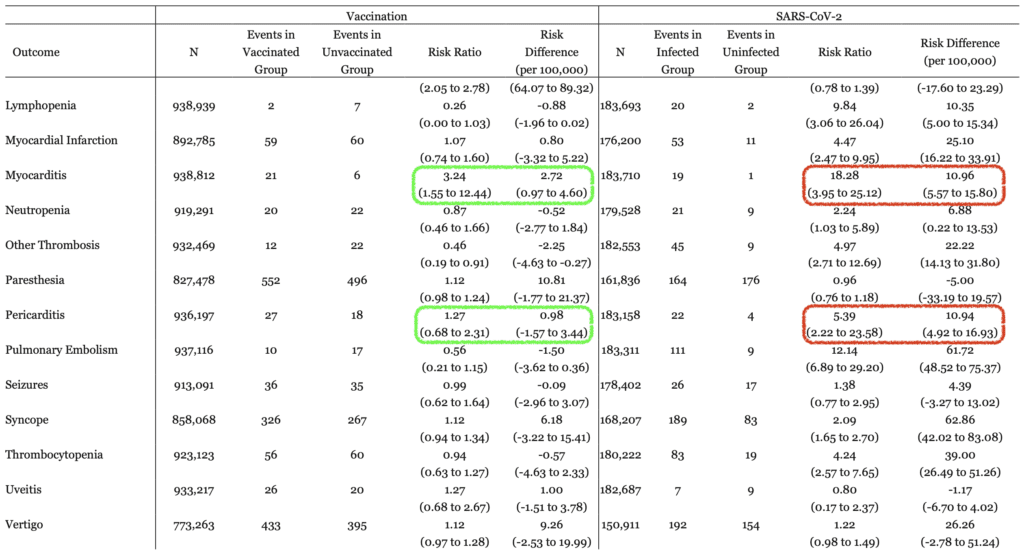
- Specifically looking at the outcome of myocarditis, there was significantly more risk of getting myocarditis in patients who were not vaccinated and acquired SARS-CoV-2 infection
- Myocarditis:
- Vaccinated RR 3.24
- Vaccinated vs Unvaccinated Risk Difference 2.72
- SARS-CoV-2 Infection RR 18.28
- SARS-CoV-2 vs No SARS-Co-V-2 Risk Difference 10.96
- Myocarditis:
Strengths:
- Asks a clinically important question
- Used a fully digitized, large (≈50% of the population) database to evaluate a broad range of potential adverse events
- Attempted to emulate a randomized assignment by having vaccinated and unvaccinated persons be exactly matched on a set of baseline variable that were deemed to be potential confounders
- No commercial funding for the study
- Drew list of potential adverse events from multiple sources (i.e. VAERS, BEST, and SPEAC, vaccine manufacturer, and relevant scientific publications)
- Gave context of risk of adverse events caused by the BNT162b2 vaccine compared to adverse events after documented infection with SARS-CoV-2
Limitations:
- The effects of vaccination and SARS-CoV-2 infection were estimated with different compositions of cohorts therefore the results of the separate sets are difficult to directly compare (i.e. Vaccination vs No vaccination and Infected vs Not infected)
- Excluded some high-risk groups (i.e. health care workers, residents in long-term care facilities) that are at higher risk of adverse events
- It is possible that people with increased clinical awareness (i.e. those getting vaccinated or after SARS-CoV-2 infection) may be more likely to report or seek medical care for their symptoms resulting in an over estimation in the incidence of various adverse events in the vaccinated or infected groups
- Adverse events such as risk of transmission to family members was outside the scope of this study
Discussion:
- The main potential adverse events associated with vaccination identified included an excess risk of:
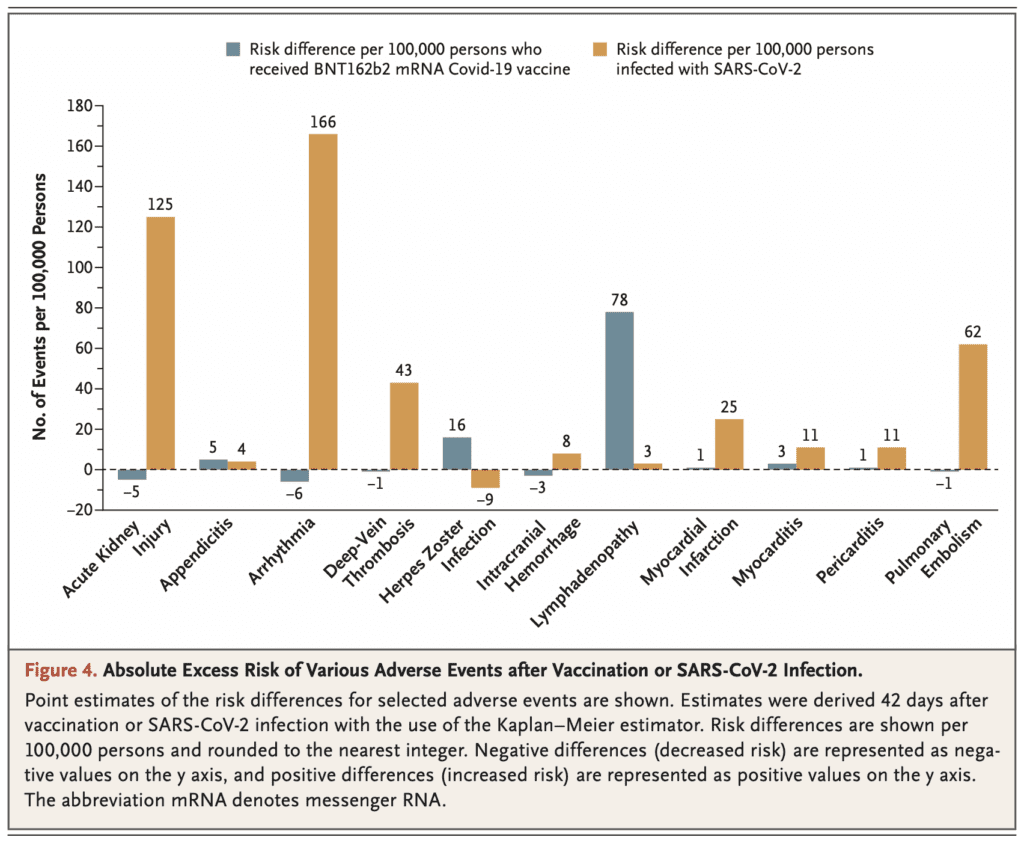
- Lymphadenopathy: 78.4 events per 100,000 persons
- Herpes Zoster Infection: 15.8 events per 100,000 persons
- Appendicitis: 5.0 events per 100,000 persons
- Myocarditis: 2.7 events per 100,000 persons
- To put potential adverse events associated with vaccination into context, it’s important to look at adverse events in persons with SARS-CoV-2 infection:
- Infection was not estimated to have a meaningful effect on incidence of lymphadenopathy, herpes zoster infection or appendicitis
- SARS-CoV-2 infection was estimated however to result in a substantial excess risk of myocarditis (11.0 events per 100,000 persons)
- Additionally, SARS-CoV-2 infection was found to substantially increase risk of several adverse events for which vaccination was not found to increase:
- Arrythmia: 166.1 events per 100,000
- Acute Kidney Injury: 125.4 events per 100,000
- Pulmonary Embolism: 61.7 events per 100,000
- DVT: 43.0 events per 100,000
- Myocardial Infarction: 25.1 events per 100,000
- Pericarditis: 10.9 events per 100,000
- Intracranial Hemorrhage: 7.6 events per 100,000
- The risk of myocarditis was significantly higher with natural SARS-CoV-2 infection (11 events per 100,000 persons) than with vaccination against SARS-CoV-2 (3 events per 100,000 persons):
- Vaccination: 3 excess events per 100,000 vaccinations
- 95% CI 1 to 5 excess events per 100,000 vaccinations
- Median age was 25 years (Range 20 to 34)
- 9% were male
Author Conclusion: “In this study in a nationwide mass vaccination setting, the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events examined. The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events was substantially increased after SARS-CoV-2 infection.”
Clinical Take Home Point: The BNT162b2 (Pfizer) vaccine for SARS-CoV-2 does result in a slight increase in incidence of adverse events over a 42 day follow up period. However, most of the adverse events are mild in nature. Myocarditis, which is considered a potentially serious adverse event, did have an increased incidence with vaccination (3 events for every 100,000 vaccinations), BUT infection with SARS-CoV-2 itself was a bigger risk for myocarditis (11 events per 100,000 infections).
References:
- Barda, N et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. NEJM 2021. PMID: 34432976
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)



