There is a lot we still do not know when it comes to COVID-19 pathophysiology. We are learning every day, and as we navigate the waters of the unknown, there are a few that boldly dare to try and understand what is happening in this disease process that may go against mainstream thinking. COVID-19 is new and therefore will require new thinking and new questions but should also be balanced with not grasping for straws and randomly doing things that could be deleterious. Below is a proposed lung injury model that may be right or could be wrong. However, the only way we can further understanding is by feedback and edits until we can get to the right answer. The purpose of this post is not to tell you what you are doing is wrong, but instead putting a model out there so that we can work on this together to find an answer. This is not a recommendation on how to treat patients, but a proposal that needs feedback and work. We felt it was a good starting place for all of us to work together to figure this thing out. Thank you to Dr. Farid Jalali, MD for putting his thoughts down on COVID-19 acute lung injury to help as a starting point.
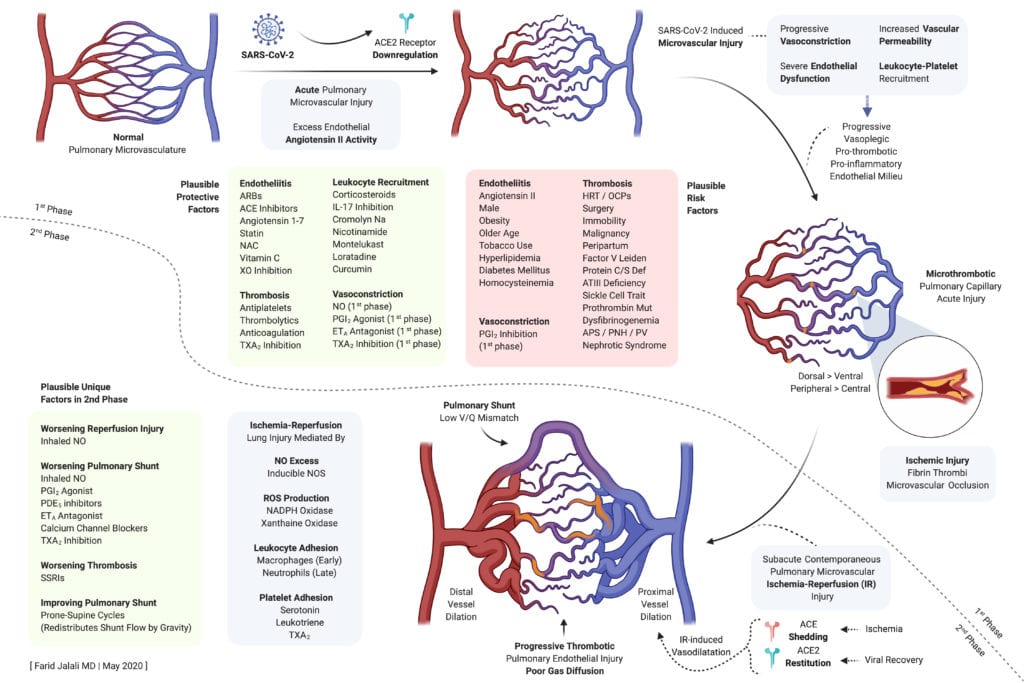
Plausible Protective and Harmful Factors
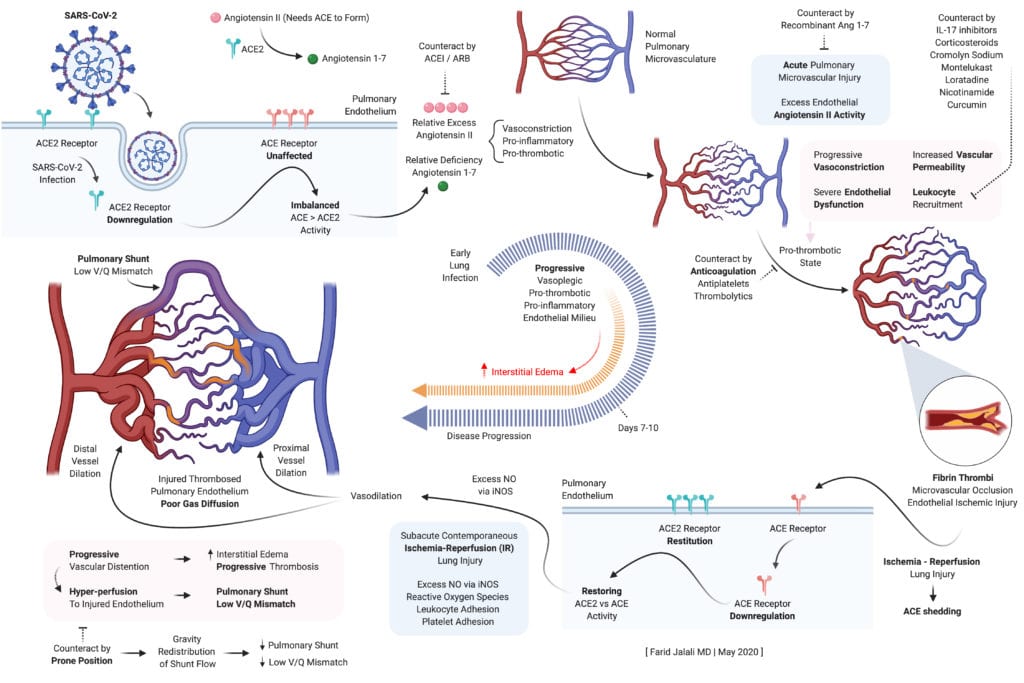
Potential Treatments at Various Stages of Disease
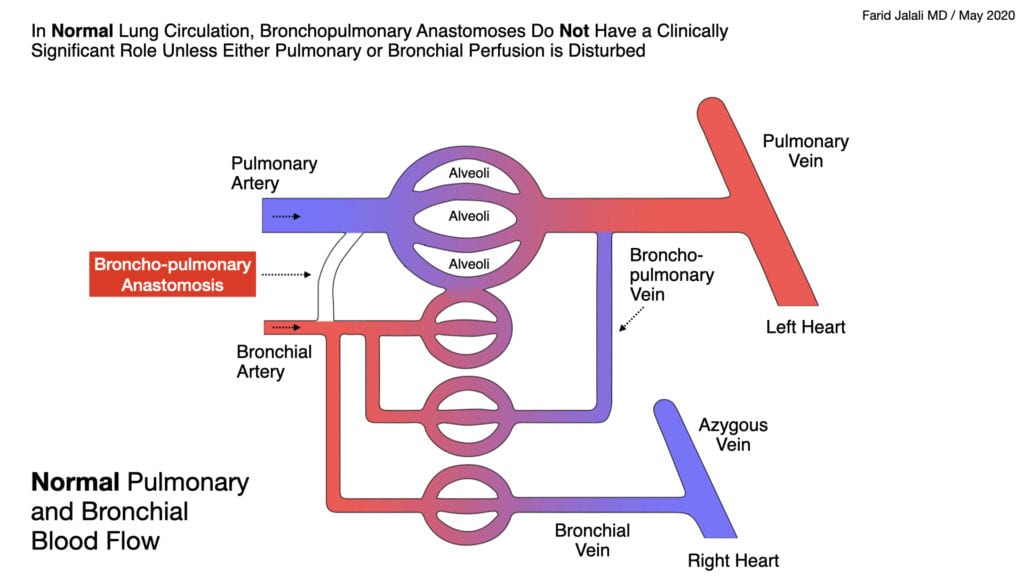
Normal Pulmonary and Bronchial Blood Flow
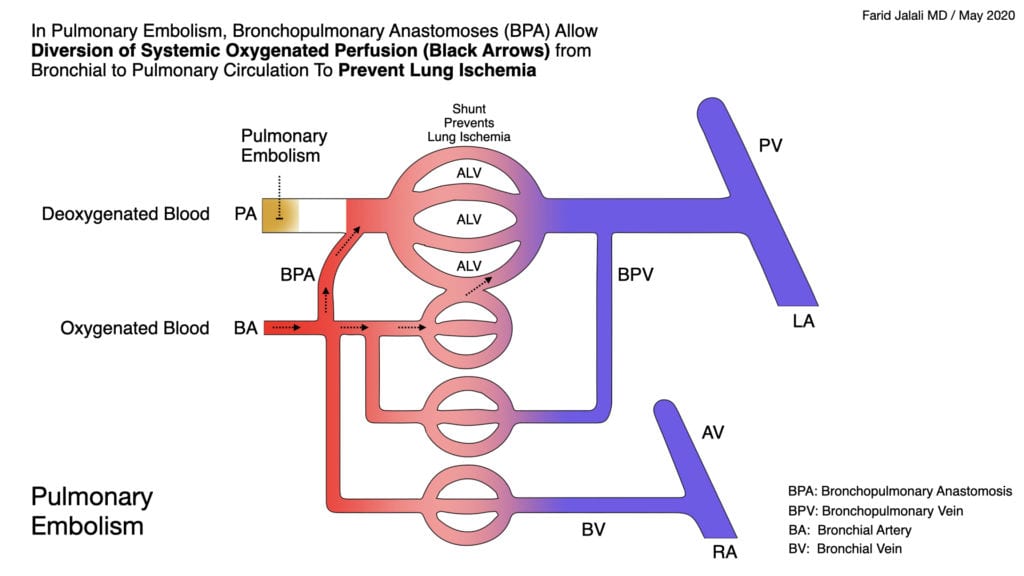
Pulmonary and Bronchial Blood Flow with Acute Pulmonary Embolism
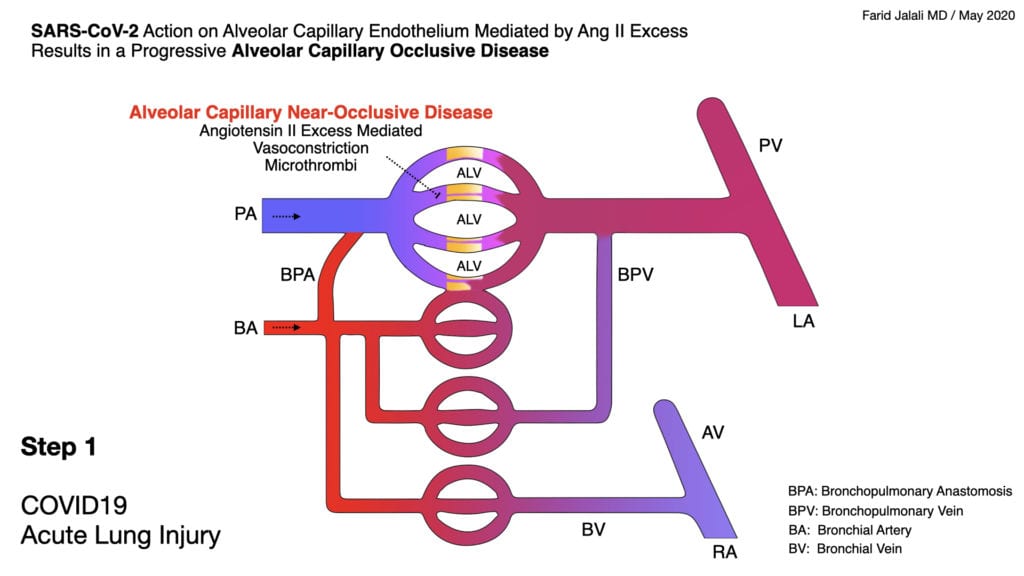
COVID-19 Acute Lung Injury Step 1
- Early endothelial stabilization, before hypoxia sets in, is key to prevent SARS-CoV-2 induced, excess Angiotensin II mediated, intense alveolar capillary vasoconstriction as well as the concomitant pro-inflammatory, pro-thrombotic endothelial milieu, all of which form the basis of lung injury in COVID-19
- Once hypoxia sets in, supportive care should include early and aggressive endothelial stabilization interventions, properly dosed anticoagulation to prevent lung microvascular thrombi, HFNC, and awake prone positioning to redistribute flow away from the forming dorsal-predominant intrapulmonary shunts
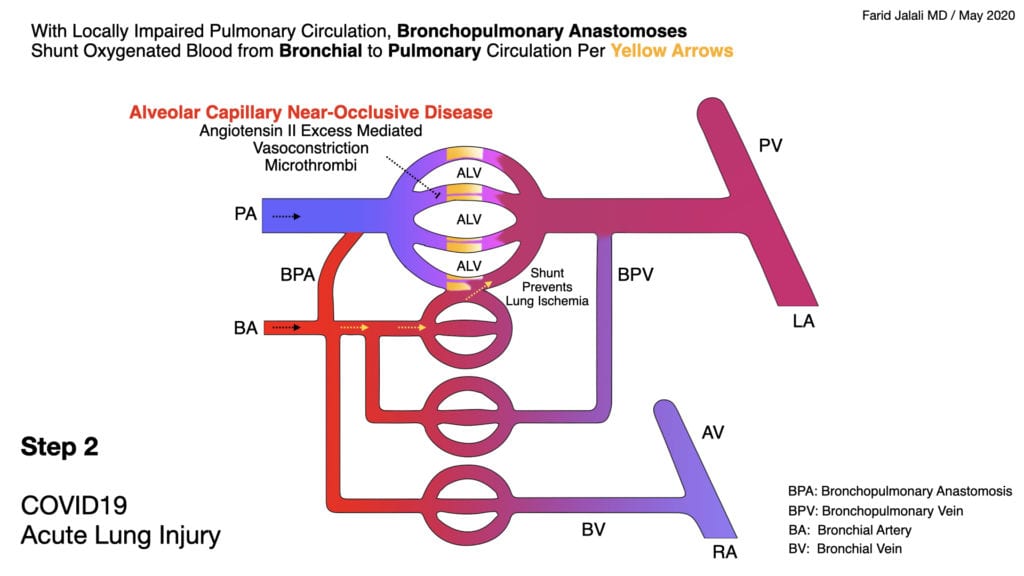
COVID-19 Acute Lung Injury Step 2
- Alveolar capillary microvascular thrombi are not a pre-requisite for the severe lung injury in COVID-19, but are a clear step in the wrong direction if allowed to be formed
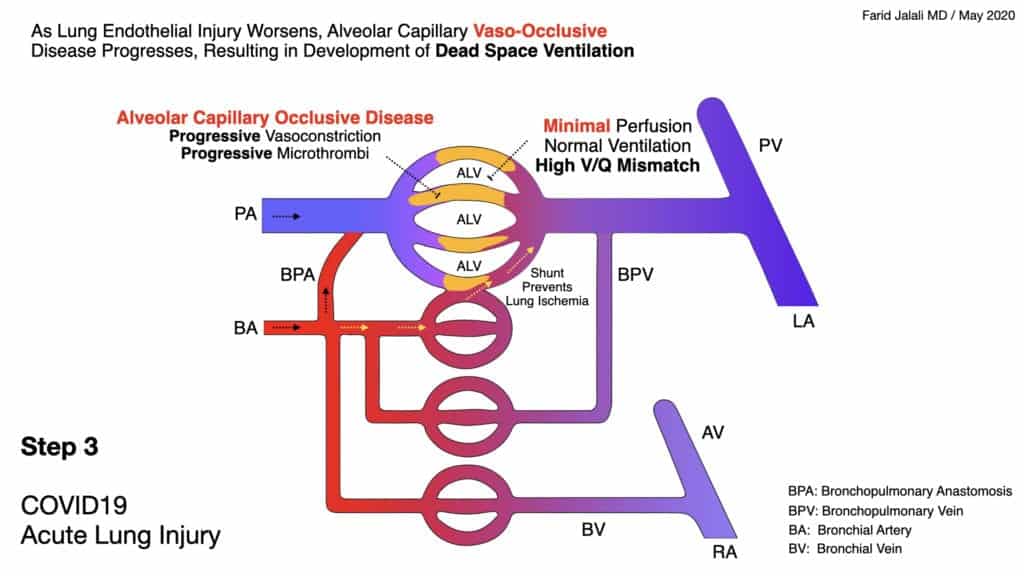
COVID-19 Acute Lung Injury Step 3
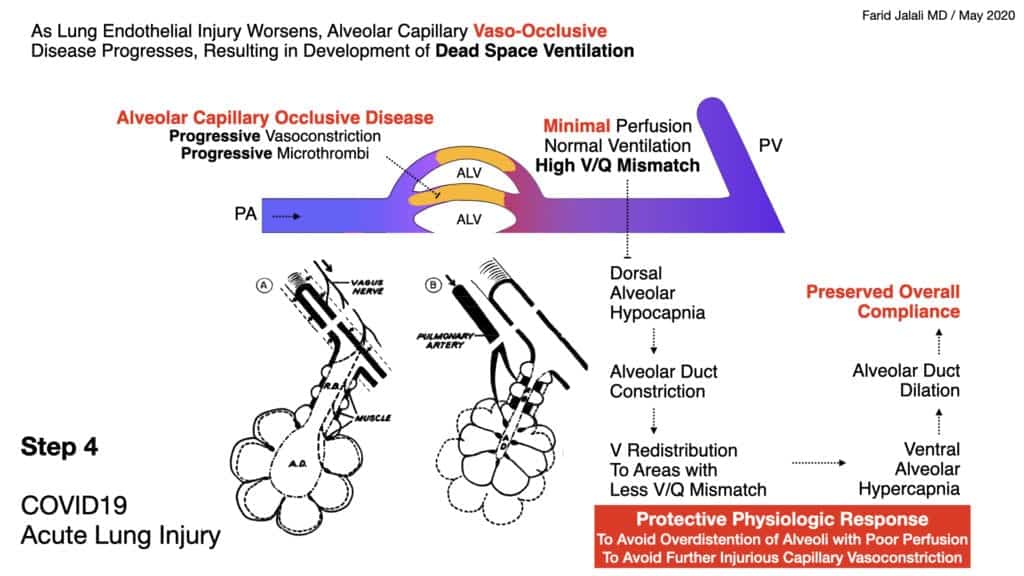
COVID-19 Acute Lung Injury Step 4
- The lung’s natural and physiologic protective response to SARS-CoV-2 induced alveolar capillary vasoconstriction and dead-space ventilation is characterized by alveolar hypocapnic bronchoconstriction at the level of the alveolar ducts to reduce a harmful alveolar hypocapnic bronchoconstriction at the level of the alveolar ducts to reduce a harmful alveolar expansion in these affected capillaries
- Naturally, unaffected capillaries and corresponding alveoli will have a higher redistribution of ventilation, will exchange more CO2 into the alveolar space, and will therefore have hypercapnia bronchodilation
- This redistribution keeps the lung compliance preserved in the initial lung injury characterized mainly by dead-space ventilation, forming intrapulmonary shunts, without significant interstitial or alveolar edema
- Compensatory lower inspiratory volumes characterize the patient’s response, associated with higher respiratory rate, and “shallow rapid breaths” without distress
- This lower inspiratory volume is needed to prevent expansion of alveoli in the affected vasculopathic areas, as inappropriate expansion compounds the vasoconstriction in these affected alveolar capillaries
- This will result in a compensatory tendency to develop hypocapnea on blood gas analysis, often concomitant with hypoxia as intrapulmonary shunts also begin to form as lung injury progresses
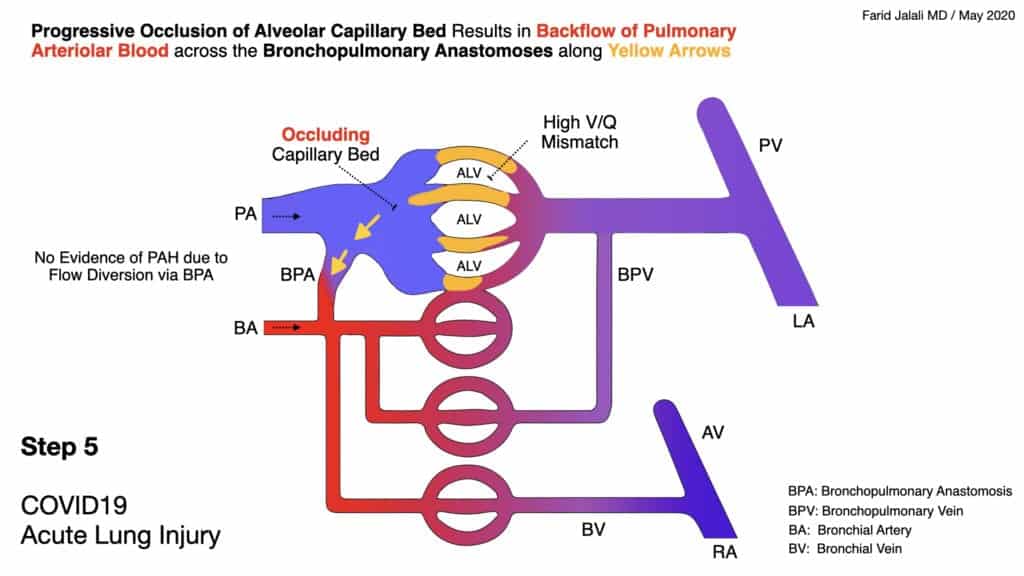
COVID-19 Acute Lung Injury Step 5
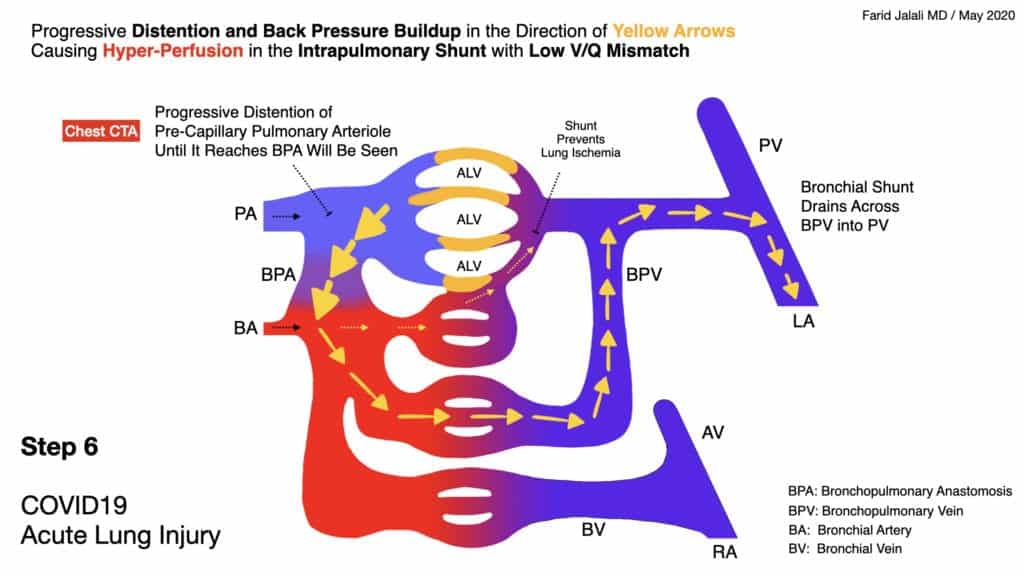
COVID-19 Acute Lung Injury Step 6
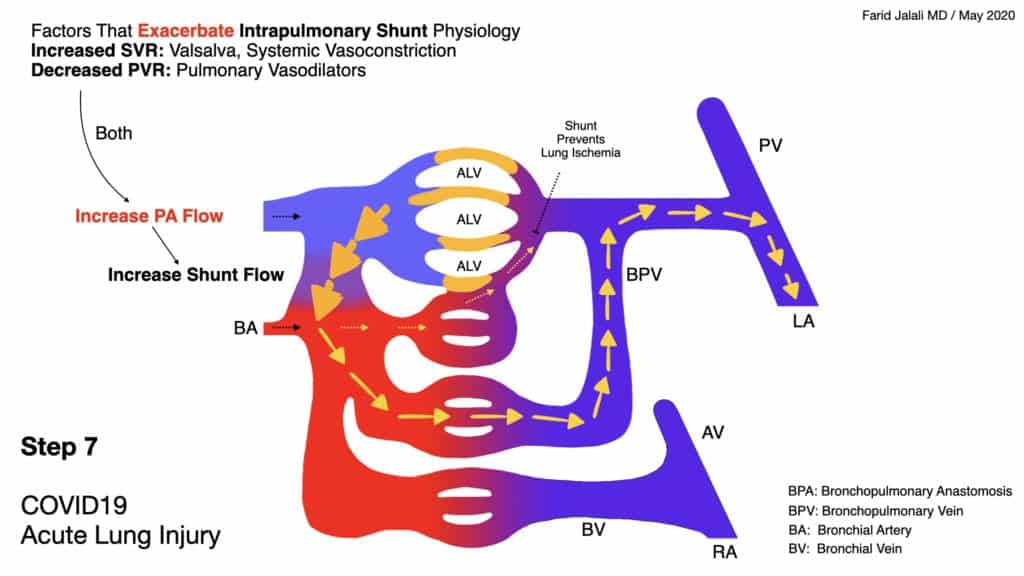
COVID-19 Acute Lung Injury Step 7
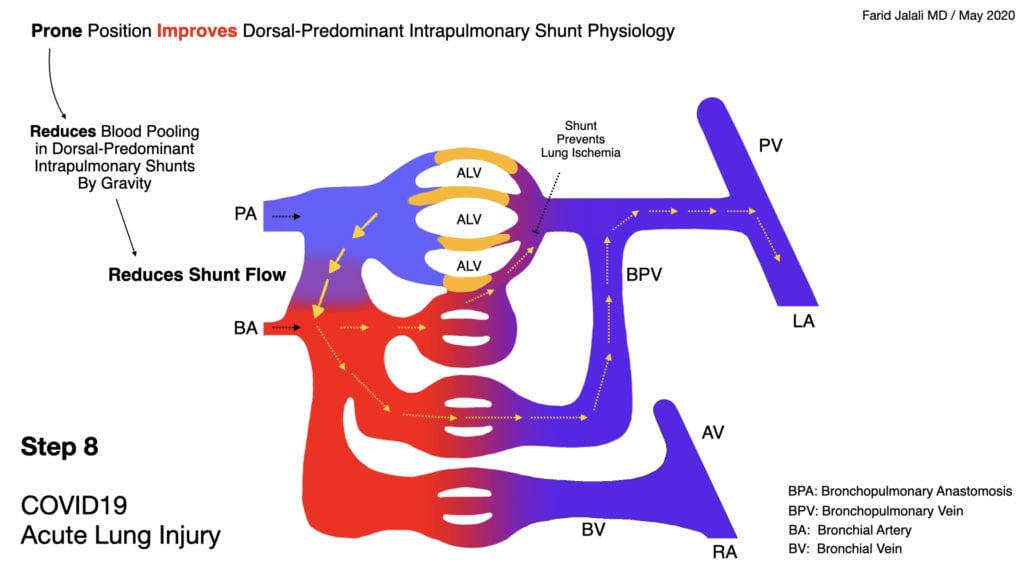
COVID-19 Acute Lung Injury Step 8
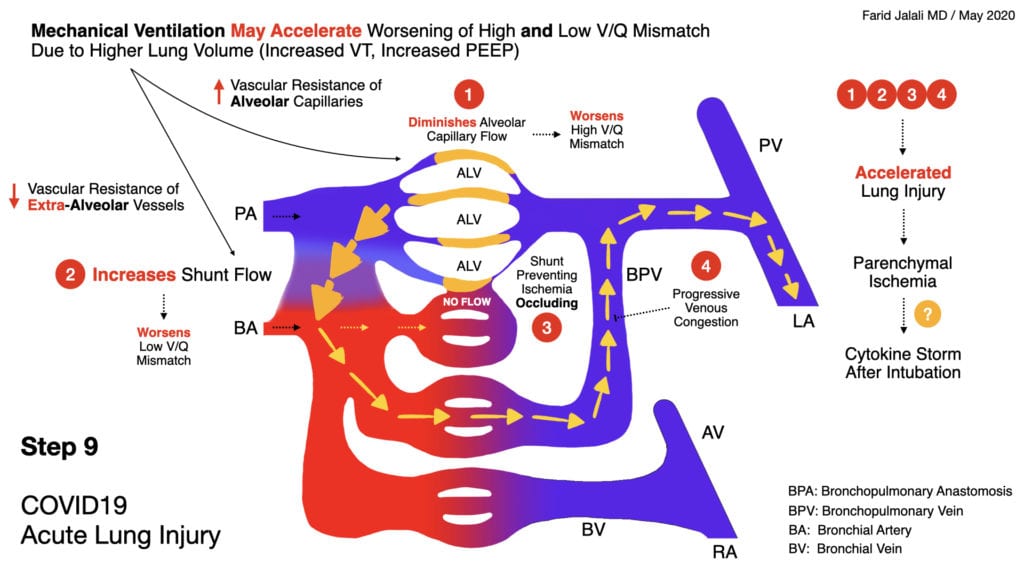
COVID-19 Acute Lung Injury Step 9
- Higher lung volumes, and positive pressure ventilation, disturb the fine balance maintained physiologically in the ventilatory redistribution pattern of the COVID-19 lung, between high V/Q mismatch areas (poor perfusion, compensatory reduced ventilation to protect against the vasculopathy) and the compensating lower V/Q areas that safely receive higher ventilation in return
- Therefore, mechanical ventilation may result in worsening of dead-space ventilation by constricting alveolar capillaries in the affected vasculopathic regions, and additionally resulting in worsening intrapulmonary shunting (next slide) due to reduced resistance in extra-alveolar vessels with higher lung volumes
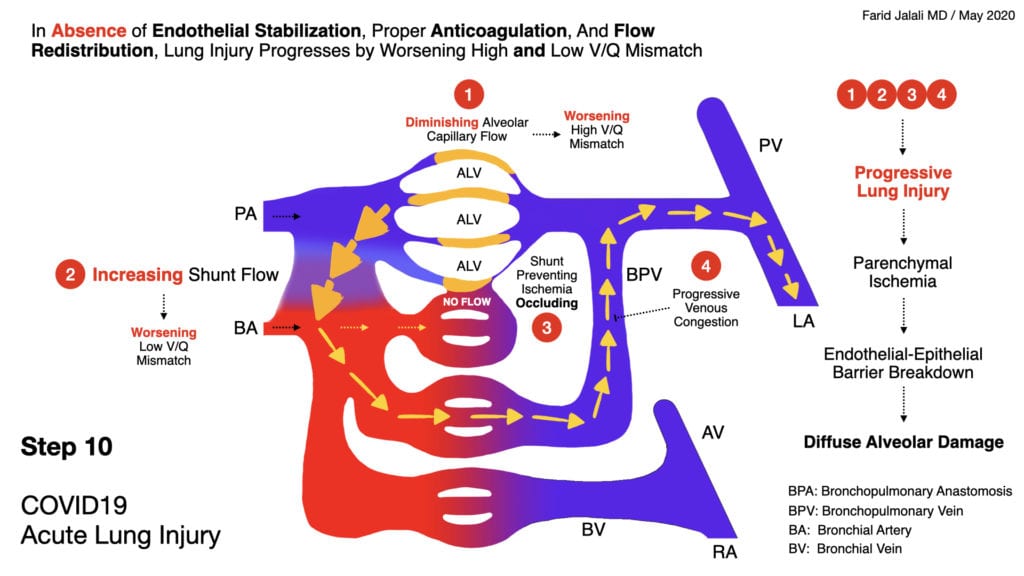
COVID-19 Acute Lung Injury Step 10
- In the absence of endothelial stabilization, proper anticoagulation, and flow redistribution, lung injury progresses to severe form by progressively worsening dead-space ventilation, resulting in intrapulmonary shunt development as described in the diagrams above
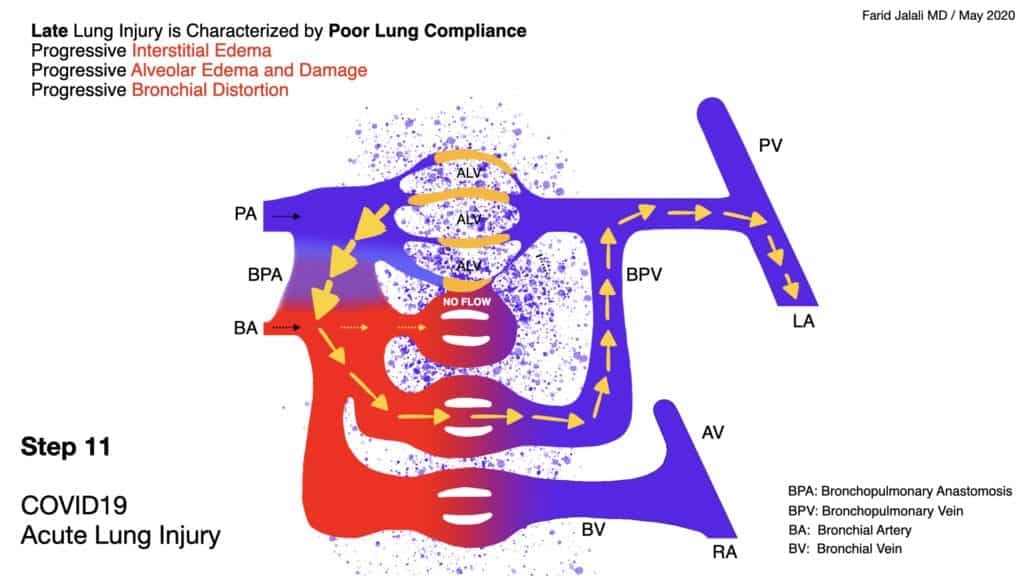
COVID-19 Acute Lung Injury Step 11
- This advanced stage of lung injury is characterized by progressively diminished flow across the alveolar capillaries, resulting in higher flow across the formed intrapulmonary shunts, eventually culminating into progressive interstitial edema, progressive and diffuse alveolar damage, and alveolar fibrin thrombi deposition
- Physiologically, this stage resembles “typical ARDS” where alveolar recruitment may be beneficial, but unlikely to reverse the vasculopathic disease process, inevitably resulting in high mortality. Pulmonary vasodilators and systemic vasoconstriction plausibly worsen hypoxia at this stage due to increasing flow across the intrapulmonary shunts
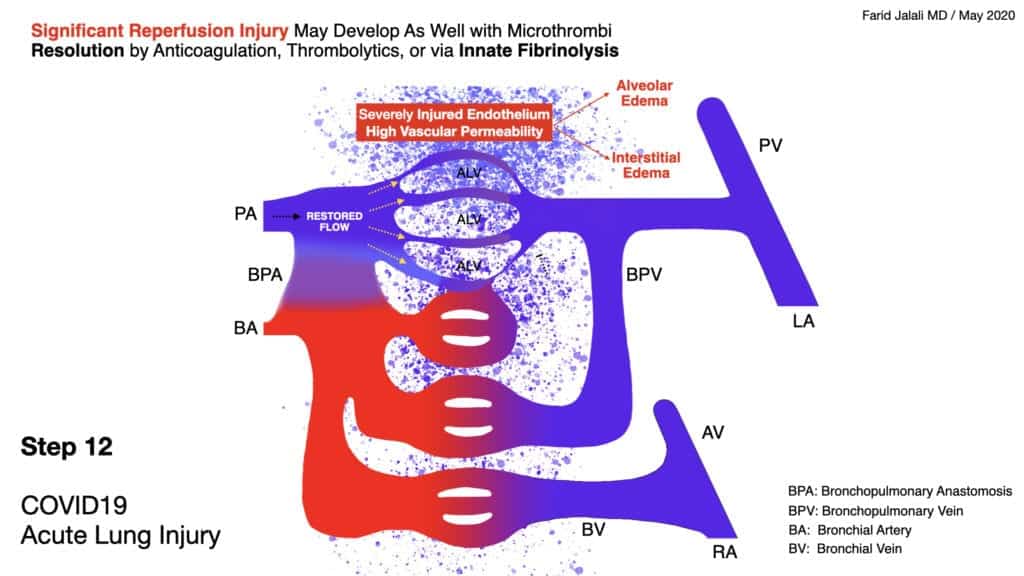
COVID-19 Acute Lung Injury Step 12
- Through the action of the body’s innate fibrinolytic system, lysis of micro thrombi and reversal of flow to an area of injured endothelium may result in cycles of ishemia-reperfusion injury in the lung, mediated early on by monocytes and macrophages, and late by neutrophil activity
- Reduction in leukocyte trafficking with corticosteroids and other therapeutics can be of value early on in the disease course to mitigate this ischemia-reperfusion injury
- Late and sudden restoration of flow to a bed of alveolar capillaries that have had a prolonged and deep poor flow usually in absence of proactive endothelial stabilization and proper anticoagulation, will inevitably result in a severe ischemia-reperfusion injury, significant interstitial and alveolar edema, and sudden demise
- At this late of a stage in lung injury, ECMO may be the only solution available while pursuing lysis of micro thrombi to restore alveolar capillary flow in a controlled fashion, while cardiopulmonary bypass is utilized to reduce risk of hemodynamic demise
[embedyt] https://www.youtube.com/watch?v=D4DwdFLWgk0[/embedyt]
Video Explaining Slides Above from Scott Weingart, MD with Farid Jalali, MD (Link is HERE)
Guest Post By:

Farid Jalali, MD
Internal Medicine – Gastroenterology
Saddleback Medical Group
Laguna Hills, CA
Twitter: @farid__jalali
PDF Version of Images: COVID-19 Acute Lung Injury A Proposed Model v1.0
Expert Commentary

Cameron Kyle-Sidell, MD
Critical Care Medicine
Emergency Medicine
Maimonides Medical Center
Brooklyn, NY
Twitter: @cameronks
I applaud Dr. Jalali for proposing a model for COVID-19 injury based on seemingly sound physiologic principles. This model does provide explanations for anecdotal observations made by bedside physicians treating COVID-19 patients. For example, the presence of dorsal-predominant shunting would explain why proning leads to marked oxygenation improvement that is not sustained once the patient returns to the supine position. If, as this model suggests, positive pressure ventilation can in fact worsen constriction to already poorly perfused capillary beds while disturbing innate ventilatory responses to V/Q mismatch (shallow breathing and alveolar hypocapnic vasoconstriction), this might explain why patients that are intubated for hypoxemic respiratory failure tend to progress to both hypoxemic and hypercapnic respiratory failure during their ICU stay.
Of course, this model, and in fact any model, beseeches us to establish the validity of those observations from which the model was partly borne. It also requires us to revisit old physiologic concepts, such as alveolar hypocapnic bronchoconstriction, which must have been lost amidst the burgeoning of material that must be taught and learned in medical education. (Never once have I heard this term spoken on ICU rounds). I hope this proposed model will lead to healthy debate over the validity of the proposed physiology, such that whether supported or refuted by new evidence, it might be remodeled as new evidence emerges.
In the end, the effort to construct a model is geared toward one aim: to better understand how to treat a disease that is afflicting the patient in front of us. This model, were it to be true, demands that we reframe some of our most important questions regarding treatment. For example, we should not just ask are ARBs, ACE-I, iNO, or other various medications beneficial for this disease, but when might they be beneficial, and when might they be harmful? And how can we determine the delineation of those stages? This model—any model—begs the difficult questions also faced by the bedside physician when the risk/benefit ratio of a therapy lacks clarity. Whether deciding to give a medication or to place on mechanical ventilation, hopefully the answers that a valid model can bring forth will eventually help us all to engage in more precise shared decision-making. That, I suspect, would be a relief to patients and providers alike.
References:
- McGonagle D et al. Immune Mechanisms of Pulmonary Intravascular Coagulopathy in COVID-19 Pneumonia. Lancet Rheumtology 2020. [Epub Ahead of Print]
- Hasegawa I et al. Bronchopulmonary Arterial Anastomosis at the Precapillary Level in Human Lung. Acta Radiologica 2020. PMID: 10598843
- KR. RK.md website: Functional Residual Capacity (FRC) and Pulmonary Vascular Resistance (PVR). 2017 [Link is HERE]
- Glowacka I et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J Virol 2010. PMID: 19864379
- Carson L et al. Pulmonary Post-Mortem Findings in a Large Series of COVID-19 Cases from Northern Italy. medRxiv pre print 2020. [Epub Ahead of Print]
- Magro C et al. Complement Associated Microvascular Injury and Thrombosis in the Pathogenesis of Severe COVID-19 Infection: A Report of Five Cases. Trans Res 2020. PMID: 32299776
- Lang M et al. Hypoxemia Related to COVID-19: Vascular and Perfusion Abnormalities on Dual-Energy CT. Lancet Infect Dis 2020. PMID: 32359410
- Albarello F et al. 2019-Novel Coronavirus Severe Adult Respiratory Distress Syndrome in Two Cases in Italy: An Uncommon Radiological Presentation. Int J Infect Dis 2020. PMID: 32112966
- Cortes-Puentes GA et al. Physiology-Guided Management of Hemodynamics in Acute Respiratory Distress Syndrome. Ann Transl Med 2018. PMID: 30370280
- Nadel JA et al. Location and Mechanism of Airway Constriction After Varium Sulfate Microembolism. J App Physiol 1964. PMID: 14173533
- Marini JJ et al. Management of COVID-19 Respiratory Distress. JAMA 2020. PMID: 32329799
- Joly BS et al. Understanding Pathophysiology of Hemostasis Disorders in Critically Ill Patients with COVID-19. Springer Link 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)



