
There is a paradigm shift towards shorter courses of antibiotics for many conditions. The investigators in this trial attempt to address the lengthy duration of antibiotics recommended and routinely prescribed among stable males with UTIs (Kang, 2018).
Article: Drekonja DM et al. Effect of 7 vs. 14 Days of Antibiotic Therapy on Resolution of Symptoms Among Afebrile Men With Urinary Tract Infection: A Randomized Clinical Trial. JAMA 2021. PMID 34313686
Clinical Question: Are seven days of antibiotics noninferior to 14 days in afebrile men with presumed UTIs?
What They Did
- Prospective, randomized, double-blinded, placebo-controlled, non-inferiority study.
- Conducted at 2 US Veterans Affairs medical centers from 2012–2019.
- Afebrile men with presumed UTI were randomized to receive seven days of antibiotics plus seven days of placebo or 14 days of antibiotics.
- Computer-generated randomization stratified by the presence of a urinary catheter, study site, and initially prescribed an antibiotic.
- Staff who enrolled and consented to patients were unaware of the treatment allocation.
Population
Inclusions
- Males, 18 years or older, treated in an outpatient setting
- Afebrile
- Prescribed 7 to 14 days of ciprofloxacin or trimethoprim/sulfamethoxazole for UTI
- At least one of the following symptoms:
- Dysuria
- Frequency of urination
- Urgency of urination
- Hematuria
- CVA tenderness
- Perineal, flank or suprapubic pain
- Up to 24 hours of inpatient observation was allowed in the study
Exclusions
- UTI treatment within 14 days
- Symptoms that clinicians attributed to non-UTI cause
- Febrile
- Growth of organism in urine that was not susceptible to the agent initially prescribed (Cipro or Bactrim).
- If the clinician opted to change the initially prescribed meds due to culture results or other parameters, the patient was considered ineligible.
Intervention
- 7 days of antibiotics followed by 7 days of placebo
Comparator
- 14 days of antibiotics
Outcomes
- Primary outcome: Resolution of UTI symptoms by day 14 after completion of active antibiotic treatment
- Secondary outcome: Recurrence of UTI symptoms within 28 days of stopping study medication
- Exploratory outcomes
- Glucose level in diabetics
- Diarrhea
- Symptom resolution in participants receiving ciprofloxacin vs. trimethoprim/sulfamethoxazole
- Symptom resolution in participants with pretreatment urinary culture and high count bacteriuria (>100,000 CFU/mL) vs. any bacteriuria vs. no bacteriuria
- Adverse events
- Differential effect of the study site (Minneapolis vs. Houston) on treatment response
Results
- 1058 screened
- 758 excluded
- Enrolled 273 patients; one was excluded
- 272 patients were randomized
- 136 patients were randomized to each arm
- 76% from the Minneapolis site and 24% from the Houston site
- 57% were prescribed ciprofloxacin, and 43% were prescribed trimethoprim/sulfamethoxazole
- The median age was 69 years
- Patients balanced for prostate issues, prior UTIs, intermittent catheter use, and indwelling catheter use
- Not balanced for the comorbid conditions of DM (10% more patients in the 14d course antibiotics)
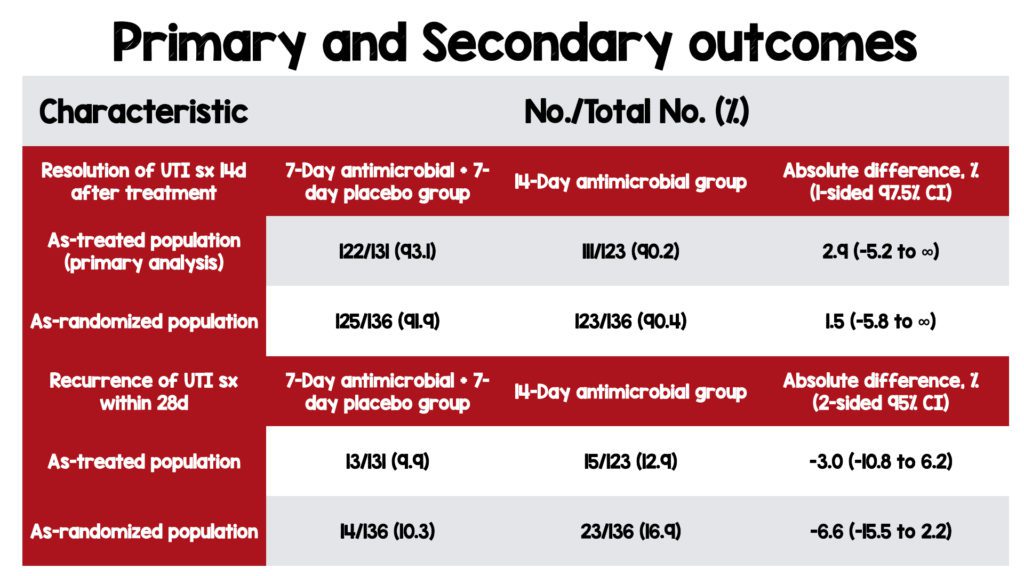
- As-Randomized Population (Intention-to-treat):
- Patients are analyzed in the group to which they are randomized whether or not they were adherent to the study protocol.
- This has many benefits, but mainly randomization remains intact.
- As-Treated Population (Per-Protocol):
- Patients are analyzed in the group to which they are randomized only if they were adherent to the study protocol.
- Investigators can assess the effect of therapy among those patients who actually received the specified treatment.
- However, randomization is lost, and groups may not be comparable due to confounders.
Critical Results
- 7 days of UTI treatment with antibiotics was non-inferior to 14 days in both the as-treated and as-randomized population.
- No difference in the recurrence of UTI symptoms in the 7-day vs. 14-day groups in both the as-treated and as-randomized population.
- No difference in exploratory outcomes.
- The results were consistent in subgroups with bacteriuria which strengthens the conclusion of shorter antibiotic courses.
- Patients stratified as high-count bacteriuria (>100 000 CFU/mL; 145 participants), any bacteriuria (184 participants), and no bacteriuria (55 participants).
- Among participants with high-count bacteriuria who received 14 days of treatment, 69/75 (92.0%) had symptom resolution vs. 65/70 (92.9%) who received seven days of treatment.
- Participants with any bacteriuria had similar results, with 83/91 (91.2%) in the 7-day group vs. 84/93 (90.3%) in the 14-day group having symptom resolution.
Strengths
- Randomized, double-blinded, placebo-controlled, dual-site trial
- Investigators asked a patient-focused research question.
- Flexible recruitment methods allowed enrollment by mail or from home (52.2% of patients), which allows patients with symptoms that occur off hours to be treated.
- Appropriately used a noninferiority study design.
- Performed an intention-to-treat and per-protocol analysis.
- No patients were lost to follow-up.
- Low rates of missing data allowed other exploratory outcomes
- Included a diverse and potentially high-risk group, including patients with indwelling catheters and intermittent catheter use.
Limitations
- Conducted in a single country study which decreases external validity.
- Patients were enrolled in the outpatient setting, which also decreases external validity and generalizability.
- The study was conducted in the VA system. It may not be generalizable to nonveterans as patients in the VA may have greater access to health care than other cohorts of the population.
- Though touted as a multicenter study, 76% of patients were enrolled from a single center.
- Appears to be a consecutive sample but overall poor enrollment.
- Homogenous population of older white males possibly decreases external validity and generalizability to other demographics.
- The placebo is only reported as being of similar size to the antibiotic. It is possible that unblinding of intervention and control group could have occurred.
- Inclusion for enrollment was subjective. Symptoms that lead to enrollment may also be seen in other conditions.
- The study protocol did not require a urine culture, but 87.9% had a pretreatment urine culture.
- Approx 1 in 4 (23%) patients with a urine culture had no bacterial growth, which may dilute any effect of treatment duration or choice of agent.
- Only two antibiotics were assessed, which decreases generalizability to patients taking other antibiotics.
- Follow-up was completed with a phone call, which may lead to recall bias
- Enrollment fell short of the planned 290 participants due to loss of funding, decreasing the power to detect a clinically significant difference.
- The noninferiority margin was based on expert opinion rather than evidence.
Discussion
- The investigators appropriately used a noninferiority trial. Longer treatment does not appear to confer additional benefits but costs more, is inconvenient for patients, potentially increases the risk of adverse events, and can lead to bacterial resistance.
- Patients were enrolled after the initiation of treatment through a search for diagnosis codes and antibiotic prescriptions. While most patients had a urinalysis (93%) and a urine culture (87%), testing was not required for enrollment. It is unclear how many patients had an abnormal urinalysis and if the results were available immediately and used to guide clinical decision-making.
- While pragmatic, enrolling patients based on symptoms is subjective and decreases confidence that the cohort has a UTI. Flank pain and hematuria may be seen in nephrolithiasis. Dysuria may be seen in sexually transmitted infections. Perineal pain may be seen in epididymitis, orchitis, or testicular torsion. Furthermore, some high-risk patients with an abnormal urinalysis may be colonized with bacteria and not acutely infected.
- Investigators studied only two antibiotics. Cipro and Bactrim are the most commonly prescribed therapies for UTI in men but the data presented is not generalizable to other antibiotic regimens. Moreover, introducing other antibiotics would have introduced heterogeneity and would likely have introduced additional barriers to achieving blinding.
- Approx 1 in 4 patients (23%) with a urine culture had no growth. Another 16% had growth lower than 100,000 CFU/mL. This may suggest that many enrolled patients did not have a UTI. However, in the sensitivity analysis, rates of symptom resolution across strata of pretreatment urine culture results (high-count bacteriuria, any bacteriuria, and no growth), there was no significant difference within each stratum. There were no significant differences in outcomes comparing short-duration vs. long-duration treatment, meaning there was no confounding in the relationship between treatment duration and success.
- Noninferiority margins are routinely arbitrarily set by consensus. The 10% safety margin seems appropriate for a group of otherwise stable afebrile men who are being treated outpatient. However, the broader the margin, the easier it is to show that one therapy is noninferior to another, especially if many patients in each arm do not have a UTI. Patient’s without a UTI may improve despite antibiotic treatment and not because of antibiotic treatment. In such cases, zero days of antibiotics may be noninferior to 14 days.
Author’s conclusion: “Among afebrile men with suspected UTI, treatment with ciprofloxacin or trimethoprim/sulfamethoxazole for 7 days was noninferior to 14 days of treatment with regard to resolution of UTI symptoms by 14 days after antibiotic therapy. The findings support the use of a 7-day course of ciprofloxacin or trimethoprim/sulfamethoxazole as an alternative to a 14-day course for treatment of afebrile men with UTI.”
Our conclusion
A 7-day course of antibiotics is noninferior to a 14-day course in afebrile men with UTIs. There was no statistically significant difference in the recurrence of UTI symptoms, and adverse events were not notably different between groups. Based on the data, there is no benefit with 14 days and no harm with seven days. Additionally, seven days of therapy is more convenient, costs less, and potentially reduces bacterial resistance compared to a 14-days course. Until more robust evidence supports a longer course, seven days is the way to go.
Clinical Bottom Line
Prescribe seven days of antibiotics In stable afebrile males with UTI.
Resources:
- Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016;57(9):485-490. PMID: 27662890
- van Nieuwkoop C et al. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med. 2017;15(1):70. PMID: 28366170
- Ulleryd P, Sandberg T. Ciprofloxacin for 2 or 4 weeks in the treatment of febrile urinary tract infection in men: a randomized trial with a 1 year follow-up. Scand J Infect Dis. 2003;35(1):34-39. PMID: 12685882
- Kang CI, Kim J, Park DW, et al. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect Chemother. 2018;50(1):67-100. PMID: 29637759
Guest Post By:

Benjamin Souferi, DO, MS
PGY-1, Emergency Medicine Resident
Vassar Brothers Hospital, Poughkeepsie, New York
E-mail: DrBenSouferi@gmail.com

Marco Propersi, DO FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)




