 Background: Welcome back to REBEL Cast episode 47. In this issue we are going to talk about some recent trials published in the past year that have gotten some love in the FOAMed world. We have been meaning to discuss these trials, but just simply didn’t have the time until now. What trials are we reviewing?
Background: Welcome back to REBEL Cast episode 47. In this issue we are going to talk about some recent trials published in the past year that have gotten some love in the FOAMed world. We have been meaning to discuss these trials, but just simply didn’t have the time until now. What trials are we reviewing?
- The age of PRBCs in transfusion
- The usefulness of lidocaine in renal colic
- The utility of oxygen therapy in Stroke
REBEL Cast Episode 47 – Blood, Kidney Stones, and Stop Using So Much Oxygen
Click here for Direct Download of Podcast
Age of PRBCs Transfused in Critically Ill: The TRANSFUSE Trial – Transfusion versus Fresher Red-Cell Use in Intensive Care [1]
Background: PRBCs can be stored for up to 42 days. During this time period cells can undergo structural and metabolic changes, which can have harmful effects in the critically ill. Usual practice is to issue oldest compatible red cells available. Prior studies, The ABLE Trial[2] (2430 critically ill patients) and the INFORM trial[3] (10,578 patients admitted to the ICU) showed no significant differences in mortality, but both trials had methodological issues limiting their conclusions.
What They Did:
- International, multicenter, randomized double-blind, parallel group trial
- 59 centers in five countries (4919 patients included in primary analysis)
- Critically ill adults received either freshest available red cells (short-term storage group) or standard issue (oldest available) red cells
- Short Term Storage (Intervention) = 11.8 +/- 5.3 days
- Long Term Storage (Control) = 22.4 +/- 7.5 days
Outcomes:
- Primary: 90d mortality
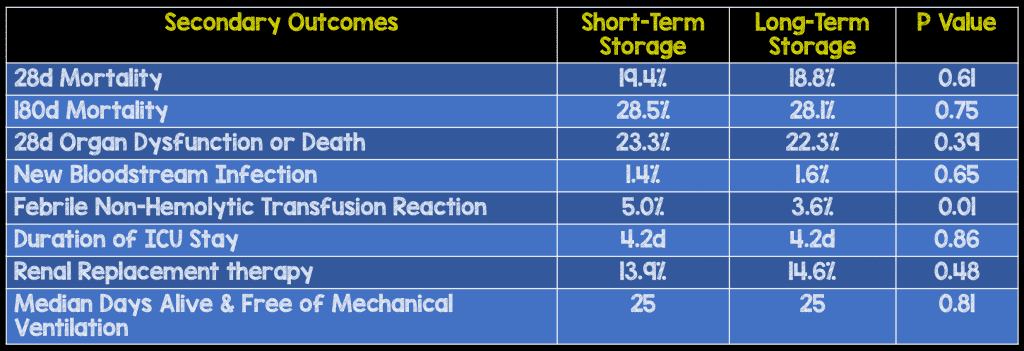
- Secondary:
- 28 day mortality
- Persistent organ dysfunction or death at day 28
- Days alive and free of renal replacement therapy at day 28
- New bloodstream infection in the ICU
- Febrile non-hemolytic transfusion reactions
- Length of stay in the hospital and the ICU
- Quality of life at day 180 after randomization
Inclusion:
- Age ≥18 years of age
- Admitted to ICU
- Anticipated ICU stay ≥24hrs
- Requirement of PRBC transfusion
Exclusion:
- Previous PRBC transfusion or cardiac surgery during hospital admission
- Hematologic cancer
- Organ transplantation
- Pregnancy
- Expected death in <24hrs
- Objection to PRBC transfusion
Results:
- 4,919 patients underwent primary analysis
- 2,457 in the short-term storage group (Mean duration of storage 11.8 days)
- 2,462 in the long-term storage group (Mean duration of storage 22.4 days)
- 90 Day Mortality
- Short-term Group: 610 patients (24.8%)
- Long-term Group: 594 patients (24.1%)
- 95% CI 01.7 – 3.1
- P = 0.57
Strengths:
- Before enrollment both the protocol and statistical analysis plans were published increasing the validity of the results
- An independent data and safety monitoring committee oversaw the trial
- There was no commercial entity support for this trial
- Randomization occurred via a concealed computer-generated schedule
- Treatment group assignments were concealed from treating medical and nursing staff, research staff, and statisticians
- All analyses were on an intention-to-treat basis and no assumptions were made for missing data
- Patient characteristics were balanced between groups
- Low rate of loss to follow up of less than 1%
Limitations:
- PRBC age in treatment groups was not prespecified but instead determined by blood-bank inventory at each institution, but very few patients received PRBCs older than 35 days of storage
- 1280 patients who were eligible for randomizations were overlooked by ICU staff and not included in the analysis
Discussion:
- All PRBCs used were leukoreduced before storage and resuspended in saline-adenine-glucose-mannitol additive solution with a 42 day shelf life (Australia and Saudi Arabia) or 35 day shelf life (New Zealand, Finland, and Ireland)
Author Conclusion: “The age of transfused red cells did not affect 90-day mortality among critically ill adults.”
Clinical Take Home Point: In this well done trial, age of transfused PRBCs had no effect on 28, 90, 180 day mortality or other clinically important secondary outcomes. Usual practice should still be transfusing the oldest compatible red cells available.
Lidocaine in Renal Colic [4]
Background: Pain is a common complaint seen in the ED, and due to the recent IV opioid shortage, providers have begun to look for other options for pain control. One such medication which has gained popularity is lidocaine. Several reasons for this increase in popularity include:
- Analgesic, and anti-inflammatory properties
- Short half-life (60 – 120 minutes)
- Predictability for adverse events.
What They Did:
- Systematic review of randomized controlled trials and observational studies using lidocaine for pain management in the ED
- Evaluate the safety and efficacy of IV lidocaine for adult patients requiring pain management in the ED
Outcomes:
- Efficacy Outcomes:
- Pain reduction
- Need for rescue analgesia
- Safety Outcomes
- Serious adverse outcomes (i.e. Cardiac arrest, seizures, dysrhythmias, hypotension)
- Non-Serious adverse outcomes (i.e. dizziness, n/v, perioral numbess)
Inclusion:
- Original research including randomized controlled trials and observational studies assessing efficacy and safety of IV lidocaine
- No restrictions on language or year of publication
- Adults ≥18 years
- Requiring pain management in the ED
Exclusion:
- Case Reports
- Receiving IV lidocaine in a setting outside the ED
- Studies using lidocaine for IV regional anesthesia (i.e. Bier Block)
Results:
- 8 Trials with 536 patients met inclusion criteria
- 6 RCTs – 2 Trials (329 patients) evaluated renal colic
- 1 Observational trial
- 1 Case series
- Efficacy Outcomes:
- Reduction in Pain Scores:
- 2 Trials considered hypothesis generating
- 2 Trials showed improved pain control (Both compared to morphine. One study on renal colic and the other on critical limb ischemia)
- 4 Trials showed no difference in pain reduction compared to active control
- Need for Rescue Analgesia: Most studies did not document which agent or doses were used
- Reduction in Pain Scores:
- 20 Adverse events (8.9%)
- 19 non-serious (Dizziness seems to be the most common)
- 1 Serious (Error in ordering and patient received 400mg of IV lidocaine resulting in seizure followed by bradycardia and brief cardiac arrest. Resuscitation successful with full neurologic recovery)
Strengths:
- Used the Cochrane Collaboration Bias Appraisal Tool (for RCTs) and a modified Newcastle-Ottawa Scale Toll (Observational studies) to evaluate risk of bias across studies
- Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach used to evaluate confidence in evidence available
- Authors of original studies were contacted for missing or unclear information/data
Limitations:
- Clinical heterogeneity and low quality of studies precluded a meta-analysis
- Active controls, causes of pain, dosing/infusion duration for IV lidocaine differed across studies making it hard to draw specific conclusions
- Significant methodological limitations, risk of bias, inconsistency, and imprecision in included studies
- Only included studies performed in the ED not other settings such as anesthesia
- Not all studies included reported all outcomes of interest
- Unclear adequacy of blinding due to predictable of effects of lidocaine
Discussion:
- We should be cautious in the use of IV lidocaine in patients over the age of 65 years and those with structural heart disease as there is no strong evidence to support its effectiveness, with the known potential harms
- Patients with cardiac disease were excluded from all the included studies and therefore we cannot apply these findings in this group of patients until better evidence is available
- None of the ED studies have used doses higher than 2mg/kg of IV lidocaine and typically given as an infusion over several minutes as opposed to IV push
- If planning on using IV lidocaine we would recommend close cardiac and vital sign monitoring during the infusion
Author Conclusion: “There is limited current evidence to define the role of intravenous lidocaine as an analgesic for patients with acute renal colic and critical limb ischemia pain in the ED. Its efficacy for other indications has not been adequately tested. The safety of lidocaine for ED pain management has not been adequately examined.”
Clinical Take Home Point: There is very limited, strong evidence supporting the use of IV lidocaine for pain control in the ED setting, however this strategy is used safely in many other settings at significantly higher doses than what is traditionally used in the ED, with essentially no harms. IV lidocaine should not be used as a first line treatment option for pain control, but could still be an opioid sparing option in patients under the age of 65 years, with no known cardiac disease in renal colic. Be cautious of dosing and be sure to use close cardiac monitoring if you plan to use.
Stroke Oxygen Study [5]
Background: In patients with ischemic stroke, the penumbra (viable brain cells beyond ischemic core) remain at risk for delayed cell death. Prophylactic supplemental oxygen could improve neurological outcomes by preventing hypoxia and secondary brain injury to the penumbra, but could also have adverse effects. The Stroke Oxygen Study (SO2S) attempted to determine whether low-dose oxygen during the first 72 hours after ischemic stroke improves outcomes when compared to usual care.
What They Did:
- Multicenter, single-blind, randomized clinical trial of 8003 patients with acute stroke
- Patients randomized 1:1:1 to:
- Continuous oxygen for 72hours (N = 2668)
- Nocturnal oxygen (9p – 7a) for 3 nights (N = 2667) – nasal oxygen impedes mobilization during the day
- Control (Oxygen only if clinically indicated; N = 2668)
- Oxygen given at 3LPM if O2 sat ≤93% and 2LPM if O2 sat >93%
Outcomes:
- Primary: modified Rankin Scale score at 90d
- Secondary:
- Neurologic improvement (≥4 point decrease on NIHSS scale) between randomization and day 7
- Highest and lowest O2 saturations during first 72 hours
- Morality at 1 week
Inclusion:
- Adults age ≥18 years
- Clinical diagnosis of acute stroke within 24 hours of hospital admission
- No clinical indications or contradictions for oxygen treatment
Results:
- 8003 patients included
- Mean NIHSS Score = 7
- Baseline Oxygen Saturation = 96.6%
- Modified Rankin Scale Score at 90d:
- No improved functional outcome at 90d when comparing Combined Oxygen vs Control (OR 0.97; 95% CI 0.89 – 1.05; p = 0.47) or Continuous Oxygen vs Nocturnal Oxygen (OR 1.03; 95% CI 0.93 – 1.13; p = 0.61)
- No difference in any of the secondary outcomes between groups
- No difference in any serious adverse events between groups
Strengths:
- Large multicenter randomized study
- Asks a clinically important question
- All three groups well balanced at baseline limiting bias of results
Limitations:
- Oxygen was discontinued prior to 72 hours in the continuous oxygen group in 16% of patients and the nocturnal oxygen group in 14% of patients and may limit our ability to find minor benefits or harms
- Main outcome was assessed by postal questionnaire and supported by telephone interviews in non-responders as opposed to in person which could lead to bias of answers and therefore the results
- The median time from stroke onset to randomization was 20hrs and 43 minutes therefore not excluding harms or benefits in early time frames
- Patients and providers were not blinded which could bias results
Discussion:
- Prior to randomization approximately 20% of the patients received oxygen in the ambulance or in the hospital
Author Conclusion: “Among nonhypoxic patients with acute stroke, the prophylactic use of low-dose oxygen supplementation did not reduce death or disability at 3 months. These findings do not support low-dose oxygen in this setting.”
Clinical Take Home Point: This is yet another study that emphasizes the fact that we should stop using oxygen in non-hypoxic patients. In patients with acute stroke, who are not hypoxemic, the use of supplemental oxygen has not shown reduction in death or disability
Clinical Bottom Line:
- Age of PRBCs has no effect on 90 day mortality in critically ill patients
- There is limited evidence supporting the use of lidocaine as a 1st line agent for pain control in the ED, however this strategy has been used safely in many other settings without harms
- In patients with acute stroke who are not hypoxemic…Hold the oxygen
References:
- Cooper DJ et al. Age of Red Cells for Transfusion and outcomes in Critically Ill Adults. NEJM 2017. PMID: 28952891
- Lacroix J et al. Age of Transfused Blood in Critically Ill Adults. NEJM 2015. PMID: 25853745
- Heddle et al. Effect of Short-Term vs Long-Term Blood Storage on Mortality After Transfusion. NEJM 2016. PMID: 27775503
- Oliveira L et al. Safety and Efficacy of Intravenous Lidocaine for Pain Management in the Emergency Department: A Systematic Review. Ann Emerg Med 2018. PMID: 29395284
- Roffe C et al. Effect of Routine Low-Dose Oxygen Supplementation on Death and Disability in Adults With Acute Stroke; the Stroke Oxygen Study Randomized Clinical Trial. JAMA 2017. PMID: 28973619
For More Thoughts on These Topic Checkout:
TRANSFUSE
Lidocaine in Renal Colic
- The Skeptics Guide to Emergency Medicine: SGEM #202 – Lidocaine for Renal Colic?
- REBEL EM: IV Lidocaine for Renal Colic – Another Opioid Sparing Option?
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



