
Also Be Sure to Checkout our YouTube Channel

This Post is Also Published on the St. Emlyn’s Blog: Beyond A(C)LS
Lets Start with a Case:
A 64 year old caucasian male has a witnessed, out of hospital cardiac arrest (OHCA). His past medical history is unknown. He receives bystander initiated CPR, intubated prehospital by EMS and brought to the ED with no pulse, last rhythm was PEA, and IV access was lost en route.
The Remainder of this Post Will Focus on going beyond ACLS and Cognitively Offloading During the Arrest in Regards to:
- CPR
- Access
- Airway
- Epinephrine
- PEA Workup
- Pulse Checks
Cardiopulmonary Resuscitation (CPR)
The 2015 American Heart Association/American College of Cardiology (AHA/ACC) published a new update for CPR quality in Circulation [1]. The key summary points for were:
- Rate: 100 – 120 compressions/min
- Depth: 2 – 2.4 inches
- Recoil: Allow Full Recoil
- Interruptions: Minimize Pauses
A review article by Cunningham LM et al [2], showed that even brief pauses in CPR can cause decreased coronary perfusion pressure (See figure below). The decreased perfusion pressure does not immediately return to adequate levels at the reinitiation of CPR. Decreased coronary perfusion pressure actually lasts longer than the pause itself and takes time to gradually build pressure back up to a baseline level, which means that cardiac tissue is actually ischemic and not being perfused for a longer period than the pause itself. This is important because ischemic cardiac tissue is less likely to be successfully defibrillated in shockable rhythms.
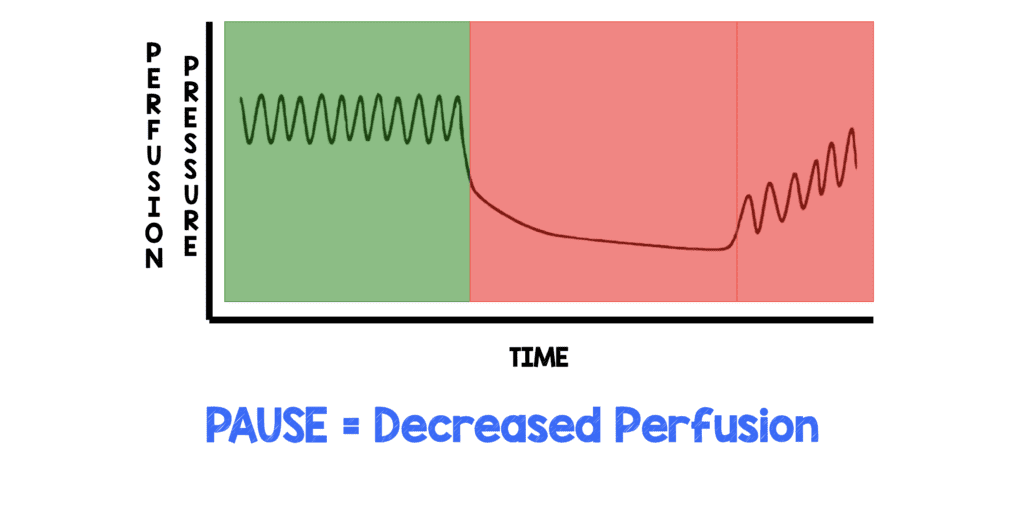
It takes up quite a bit of mental bandwidth to ensure that CPR is performed well and there is already enough going on during the resuscitation, so is there a way that we can cognitively offload our minds?
There was a systematic review and meta-analysis of randomized clinical trials trying to answer the question of mechanical CPR vs manual CPR in OHCA by Gates S et al in Resuscitation 2015 [3]. The trial included 5 randomized clinical trials (RCTs) with over 10,000 patients with OHCA. Ultimately, the study found no difference in ROSC, Survival, or Survival with Good Neurologic Outcomes. None of the studies showed superiority of mechanical CPR, but also none of the studies showed inferiority to manual CPR. In other words, mechanical CPR to date is no better or worse than manual CPR.
Clinical Bottom Line: Doing high quality uninterrupted CPR is important, but this is not the endpoint of resuscitation. Mechanical CPR is a great way to off load our minds. We now don’t have to focus on the rate of compressions, depth of compressions, interruptions in compressions and instead focus on why our patient has had a CA.
Our Patient: Our patient gets mechanical CPR initiated, but remember EMS lost IV access…
Access
I recently asked the twitterverse, in a patient with CA and no IV Access, what is your go to access? The results are below:
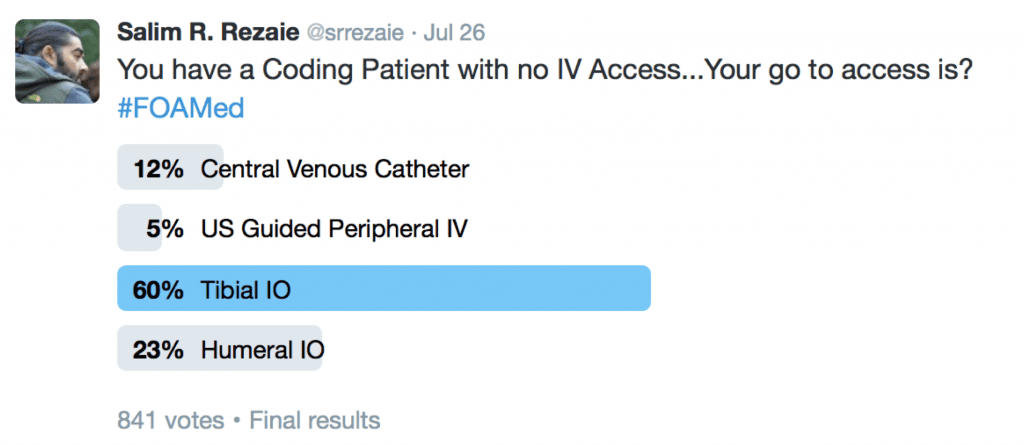
In my mind there are really only 2 sites for intraosseous (IO) access during CA: Humeral Head or Proximal Tibia. Sternal IO has been shown to give a faster infusion rate than other sites, but may get in the way of CPR in CA [10]. The proximal humerus is closer to the heart and thus gets medications circulating faster than the proximal tibia. On the other hand the proximal tibia is away from the head of the bed which is where most people are typically congregated for airway management and CPR. Another way to think about the IO sites of access are:
- Humeral Head and Sternal IO = Central Venous Catheter (Closer to the Heart)
- Tibial IO = Peripheral Intravenous Access (More Distal to the Heart)
There was a RCT done by Reades et al in Ann Emerg Med 2011 [4], which is the largest RCT to date comparing humeral IO vs tibial IO vs peripheral IV (PIV). This study included 183 patients with OHCA. Their primary outcome was 1st attempt success at access and a secondary outcome was time to access.
- First Attempt Success:
- Tibial IO: 91%
- Humeral IO: 51%
- PIV: 43%
- Time to Access (Min)
- Tibial IO: 4.6
- Humeral IO: 7.0
- PIV: 5.8
- A Huge caveat to this trial, is that this was a prehospital study, and not a study in the ED setting. Secondly there may have been some issue with familiarity in the placement of humeral IOs as is evidenced by the time to access (7 minutes?!?).
Clinical Bottom Line: When having difficulty getting IV access during CA, humeral IO has a higher success rate and faster time to access being achieved vs IV
Our Patient: Receives two 15 gauge Proximal Humerus IOs and several people begin to ask about airway management
Airway
AIRWAYS-2 [11]
What They Did: This was a multicenter, cluster*randomized clinical trial of paramedics from 4 ambulance services in England. The objective of the study was to determine whether a supraglottic airway device (SGA) is superior to tracheal intubation (TI) as the initial advanced airway management strategy in adults with non-traumatic OHCA
- *Interestingly, randomizing patients at the point of OHCA was considered impractical, therefore, paramedics were randomized to use 1 of 2 advanced airway management strategies for the eligible patients that they treated
- SGA = Second-generation supraglottic airway device with a soft non-inflatable cuff (i-gel; intersurgical)
- TI = Direct laryngoscopy as video laryngoscopy not used by paramedics in England
- 9,296 patients enrolled into trial
- SGA Group = 4886
- TI Group = 4410
- Good mRS Score (0 – 3) at Hospital DC or 30 days:
- SGA = 6.4%
- TI = 6.8%
- Adjusted RD -0.6%; 95% CI -1.6% – 0.4%
- 1,707 of patients received no advanced airway management
- Survival with Good Neuro Outcome
- TI but No Advanced Airway Management = 21.6%
- SGA but No Advanced Airway Management = 20.5%
- Survival with Good Neuro Outcome
- The use of advanced airway management was greater among paramedics in the supraglottic airway device group (85%) compared with those in the tracheal intubation group (78%), which could result in confounding by indication as well.
- The use of supraglottic airway devices as the first advanced airway technique in OHCA was associated with better outcomes, however the between group differences were less than the pre-specified clinically important difference and less than the minimally important difference of ≈3% reported in other studies.Therefore this is a hypothesis generating conclusion.
- A paper by Jabre P et al [12] comparing BVM vs TI for airway management during OHCA in >2000 patients in France and Belgium. The primary outcome was favorable neurological outcome at 28 days. There was essentially no difference in this outcome between groups (4.3% vs 4.2%; 95% CI -7.7% – 0.3%).
- Clinical Bottom Line: Among patient with OHCA, it appears the key to airway management seems to be choosing a strategy that doesn’t get in the way of the things that matter most to achieve better survival with good neurologic outcomes (i.e. high quality CPR).
Our Patient: Gets an Supraglottic airway (SGA and now several people in the room begin to call for epinephrine (adrenaline)
Epinephrine Dosing/Timing
What does the twitterverse feel is the correct dosing and timing of epinephrine in CA:
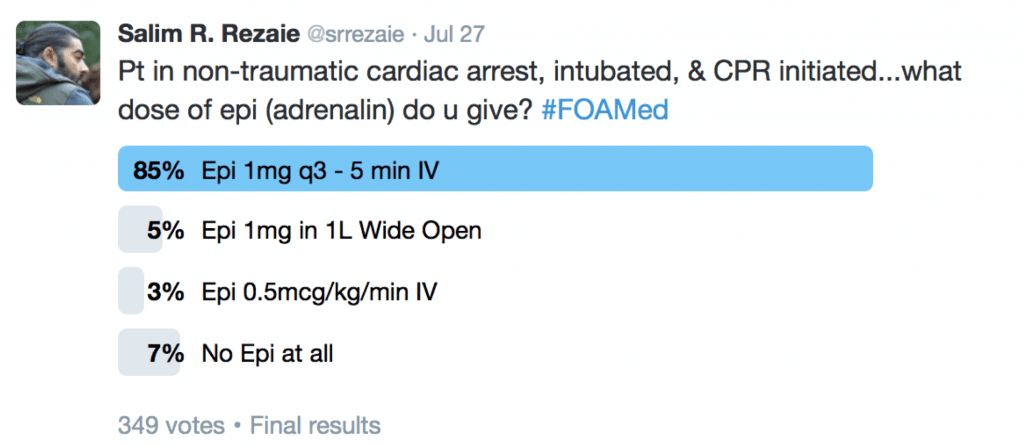
The 2015 Adult Advanced Cardiac Life Support (ACLS) Guidelines recommend that it MAYBE reasonable to give epinephrine 1mg IV q3 – 5 minutes maybe reasonable [5]. They give this a Class IIb recommendation, which is another way of saying weak evidence (Benefit ≥ Risk).
Many studies looking at outcomes with dosing epinephrine as recommended by ACLS look at surrogate outcomes, specifically ROSC. In CA, ROSC is the first step, but what we really care about is neurologically intact survival. To date, there is no study that has shown epinephrine in CA improves neurologically intact survival.
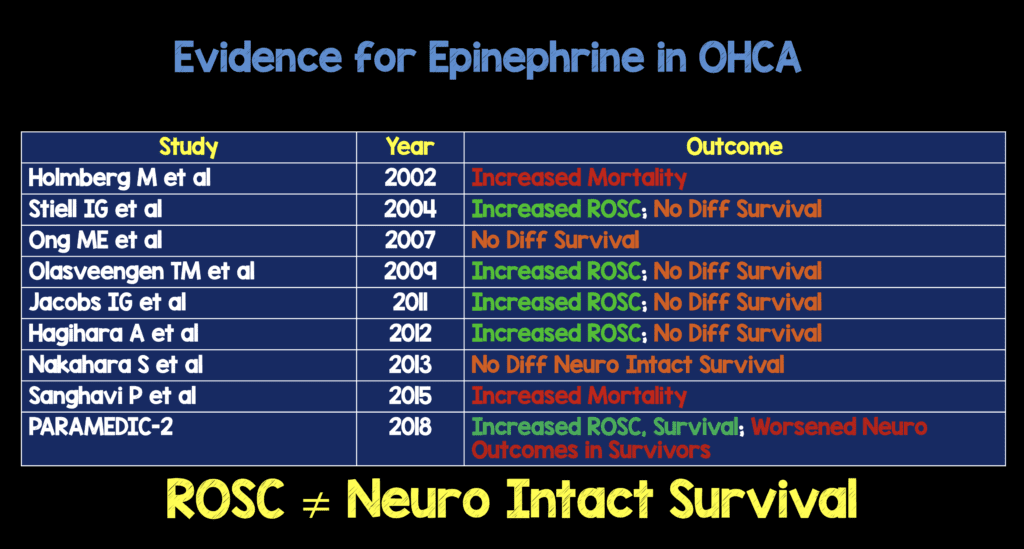
Epinephrine does produce some beneficial effects in CA of increased coronary perfusion pressure and cerebral perfusion pressure during CPR, but in bolus doses may increase myocardial oxygen consumption and therefore increase subendocardial ischemia.
PARAMEDIC-2 [13]
What They Did: Prehospital Assessment of the Role of Adrenaline: Measuring the Effectiveness of Drug Administration in Cardiac Arrest (PARAMEDIC-2).This was a multicenter, randomized, double-blind, placebo controlled trial involving 8014 patients with OHCA in the United Kingdom
- 8014 patients with OHCA
- 4015 in the IV epinephrine arm (Epi)
- 3999 in the IV saline placebo arm (Placebo)
- Survival to Hospital DC with Favorable Neurologic Outcome
- Epi: 2.2%
- Placebo: 1.9%
- uOR 1.1
- Severe Neurologi Impairment (mRs 4 – 5)
- Epi 31.0%
- Placebo 17.8%
- Clinical Take Home Point: In this well done, multicenter trial epinephrine given at 1mg q3 – 5 minutes increased the chances of ROSC but came at a cost of more ICU usage, more patients with severe neurological disabilities, and no difference in survival with favorable neurological outcome compared to placebo. The use of epinephrine 1mg q 3 – 5 minutes should no longer be part of standard cardiac arrest protocols. Epinephrine should be administered on a case by case basis by experienced providers who perceive that there is a benefit to be had.
Now I will be the first to admit, it can cause undue unrest in the dynamics of your team during the resuscitation if you recommend skipping the epinephrine entirely without a very lengthy explanation. Instead, I have two other options to consider that will remove the distraction of remembering epinephrine every 3 – 5 minutes:
- Option 1: The Dirty Epi Drip – Push 1mg (i.e. 1 ampule) of code cart epinephrine into 1,000mL of NS which gives you a final concentration of 1mcg/mL. You can run this wide open with a pressure bag until, a) your patients hemodynamics stabilize, or b)an epinephrine drip is ready for use.
- Option 2: Quarter Dose Epi Drip: Run epinephrine at 0.5mcg/kg/min. In a 100kg patient this would be the equivalent of 250mcg over 5 minutes (i.e. quarter dose)
Running an epinephrine drip allows for several things to occur:
- Cognitive Offload: No one has to remember, “is time for the next epi,” every 3 – 5 minutes
- Minimizing Physiological Lability: Continuous dose of epinephrine allows for smoother coronary and cerebral perfusion pressure without increased myocardial oxygen consumption and subendocardial ischemia (This is my contention, but no studies comparing epi drip vs standard 1mg epi dosing q3 – 5min in CA)
- Avoidance of post ROSC Drop in Blood Pressure: Titrate the drip to the dose needed
Clinical Bottom Line: No study to date has shown that the ACLS recommended 1mg epinephrine q3 – 5 minutes improves neurologically intact survival in CA. Maybe a better way would be to run a continuous infusion of lower dose epinephrine.
Our Patient: Starts with a Dirty Epi Drip and is transitioned to a Quarter Dose Epi Drip
PEA Workup
Approximately 1/3 of patients in CA have PEA and it is well known that patients with PEA as an initial rhythm have a worse survival than patients with a shockable rhythm [6]. If you are like me you learned all about the H’s and T’s that could potentially cause PEA, but even now try and list all of them. Now imagine yourself trying to go through this differential while resuscitating a patient in PEA CA. Pittman et al published an interesting article in Med Princ Pract 2014 [6] which focused on the few causes of PEA that we can rapidly and effectively correct instead of memorizing the traditional H’s and T’s. The steps in their algorithm are simple:
- Determine if the PEA is narrow (QRS Duration <0.12) or wide (QRS Duration ≥0.12)
- Narrow-complex PEA is generally due to mechanical problems caused by right ventricular inflow or outflow obstruction
- Wide-complex PEA is generally due to metabolic problems
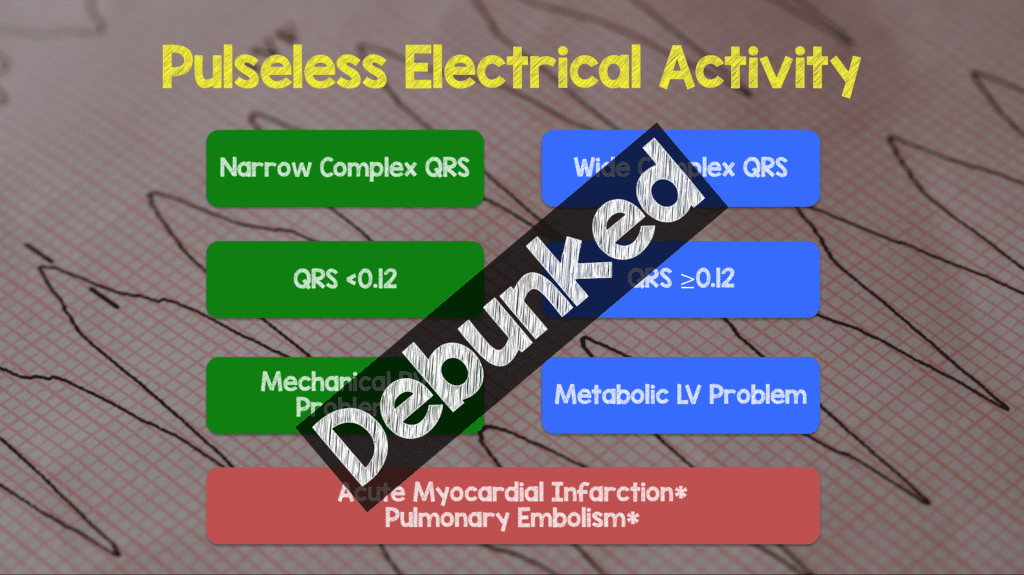
- A subsequent study [14] showed that QRS width was not being a predictor of cause of PEA. In the follow up study 51 patients with IHCA and PEA arrest were prospectively observed. The bottom line was no unique ECG patterns were associated with underlying cause of PEA (Consider this theory debunked).
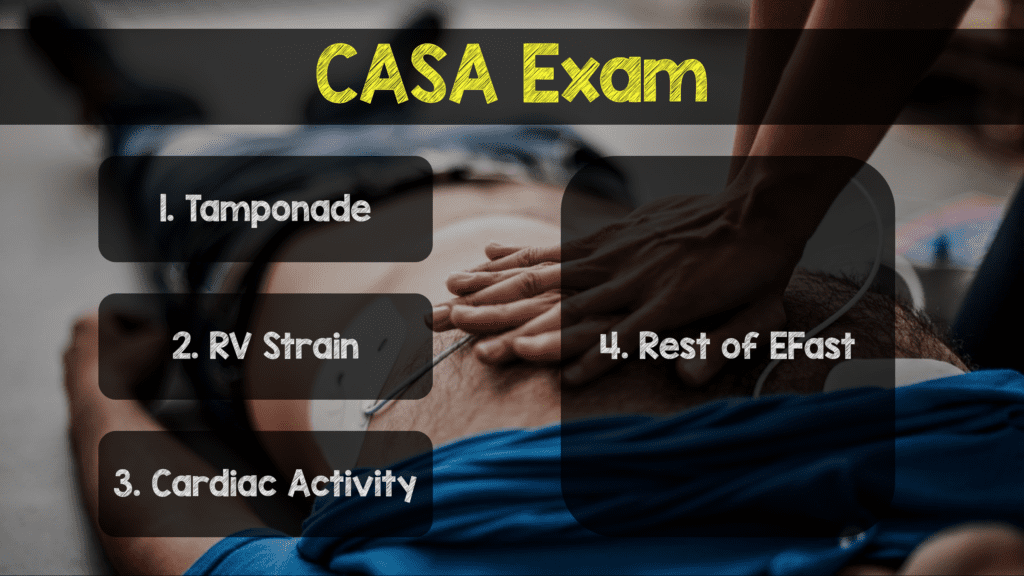
- The CASA Exam [15]
- What They Did: Description of a simplified POCUS algorithm during cardiac arrest. The exam consists of three, <10second POCUS exams during rhythm checks as shown in the image below. A phased array probe used in the subxiphoid view is the optimal view suggested as this view does not interrupt CPR. A key point is to have someone count out loud to not prolong CPR pauses and to record while ultrasounding so that images may be reviewed after resuming CPR. Finally, E-FAST can be performed on a case by case basis according to the authors.
- Step 1: According to the authors pericardial effusion causing cardiac tamponade is the cause of cardiac arrest in 4 – 15% of patients. Tamponade can be intervened upon with the use of pericardiocentesis.
- Step 2: The authors state that, pulmonary embolism is the cause of cardiac arrest in 4.0 – 7.6% of patients. Evidence of right heart strain, should place pulmonary embolism higher in the differential, but there can be other causes of right heart strain. If PE is diagnosed, systemic thrombolysis is recommended.
- Step 3: Finally, the authors state that the presence of absence of cardiac activity can provide useful prognostic information. Obviously the absence of cardiac activity in PEA portends worse outcomes (survival to hospital discharge rate = 0.0% – 0.6%). This finding along with other poor prognostic factors such as low end-tidal CO2, prolonged downtime, and unwitnessed arrest can help in the utility of ongoing resuscitation.
- Ancillary Steps: Tension pneumothorax is a rare cause of non-traumatic cardiac arrest, but can be assessed by absence of lung sliding on POCUS in the anterior chest. If detected, needle decompression or finger/tube thoracostomy may be considered
- Clinical Take Home Point: POCUS has become one of the most important tools in discovering both the diagnosis and in the management of critically ill patients. Newer literature, however indicates that the use of POCUS prolongs CPR pauses which ultimately impacts good neurological survival. POCUS protocols such as the CASA exam may help decrease cognitive load and minimize pauses.
Our Patient: Our patient gets the CASA exam and bedside ultrasound finds a dilated RV > LV and concern for a massive PE. The patient receives systemic tPA and has a change in the rhythm on the monitor. Someone calls out, can you feel a pulse?
Pulse Checks
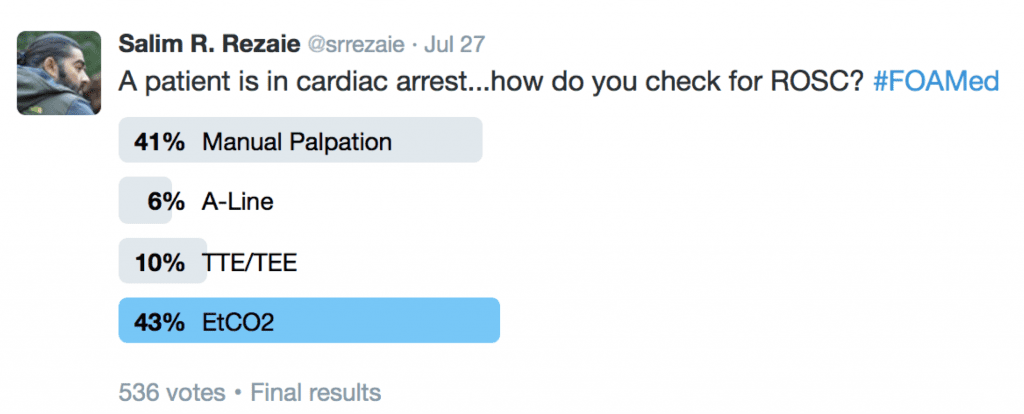
What does the twitterverse feel is the best way to check for ROSC in CA? The results are below:
Ochoa FJ et al published a trial in Resuscitation 1998 [7] in which ED physicians, ICU physicians, and nurses tried to identify a carotid pulse in a healthy male volunteer with normal blood pressure. 43.1% of health professionals required >5secs to detect the carotid pulse and 4.3% required >10secs.
So is the manual pulse check dead? Maybe. I certainly think we have better technologies to assist in determining ROSC that don’t require pauses in CPR:
- Arterial Line: The benefit of the arterial line is we get direct feedback on cardiac output, but the downside is it takes time to place
- Bedside Transthoracic Echocardiogram (TTE): This gives the provider real time feedback of what the heart is actually doing (i.e. Not feeling a pulse manually, when in reality the heart is weakly contracting – Pseudo PEA), can also give real time CPR quality feedback, and potentially has the ability to predict the chances of ROSC [8]. The downside is we still have to stop CPR to look for cardiac activity, but if done correctly should not take >10secs to see if the LV is contracting or not. Blyth L published a systematic review of patients in CA in Academic Emergency Medicine 2012 [8]. This included 8 studies with 568 patients with OHCA and who had a bedside echocardiogram performed. At 20 minutes if there was cardiac motion on echo 51.6% of patients achieved ROSC and 48.4% did not achieve ROSC. On the other hand, if there was no cardiac motion at 20 minutes on bedside echo only 2.4% achieved ROSC while 97.6% did not. One major issue with this study is ROSC was used as a surrogate marker, and neurologic outcomes were not assessed.
- End Tidal CO2 (EtCO2): For me, this is where the money is at. Ahrens T et al looked at EtCO2 in OHCA as a prognostic indicator of outcomes [9] and found that a EtCO2 <10 – 15mmHg at 20 minutes predicted a 0.8% chance of survival. The studies evaluating the use of EtCO2 in CA have been in intubated patients, observational studies, and to date none have investigated survival with intact neurologic outcome. The 2015 AHA/ACC guidelines actually state that in intubated patients, failure to achieve an EtCO2 of >10mmHg by waveform capnography after 20 minutes of CPR maybe considered as one component of a multimodal approach to decide when to end resuscitative efforts, but should not be used in isolation (ClassIIb)[5]. Currently there is no optimal duration of resuscitation before the termination of resuscitation (TOR).
- Lungs require ventilation and perfusion to work. If we keep ventilation constant with a bag valve mask or ventilator then perfusion is the only factor not accounted for. Therefore an increase in cardiac output should increase the EtCO2 reading. However, anything that interrupts ventilation will cause false readings of EtCO2 (i.e. Dislodged Endotracheal Tube, Respiratory Causes of CA, and Pulmonary Embolism)
Clinical Bottom Line: Stopping CPR for pulse checks is not accurate at predicting ROSC and potentially detrimental to neurologic outcomes of patients. Adjuncts such as bedside echocardiography and EtCO2 add to a multimodal approach in predicting ROSC as well as giving useful information in decisions of termination of resuscitation.
Our Patient: Had EtCO2 placed and after thrombolytics had a jump in his EtCO2 to 30mmHg and found to have ROSC on bedside Echo
Critical Take Home Points to go Beyond ACLS and Cognitively Offload During Resuscitation Efforts of Cardiac Arrest:
- CPR: Mechanical CPR > Manual CPR
- Access: IO Access > IV Access
- Airway: SGA > BVM > ETI
- Epinephrine: Epinephrine Drip > Epinephrine Bolus
- PEA Workup: POCUS (CASA Exam) > H’s & T’s
- Pulse Checks: EtCO2 + Bedside TTE > Manual Pulse Checks
For More on This Checkout:
- Jonathan St. George at The Ember Project: The Lucas Device and Cognitive Offloading
- Anton Helman at EM Cases: Episode 96 – Beyond ACLS Cardiac Arrest – Live from EMU Conference 2017
References:
- Kleinman ME et al. Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132 (18 Suppl 2): S414 – 25. PMID: 264722993
- Cunningham LM et al. Cardiopulmonary Resuscitation for Cardiac arrest: The Importance of Uninterrupted Chest Compressions in Cardiac Arrest Resuscitation. Am J Emerg Med 2012; 30(8): 1630 – 8. PMID: 22633716
- Gates S et al. Mechanical Chest Compression for Out of Hospital Cardiac Arrest: Systematic Review and Meta-Analysis. Resuscitation 2015; 94: 91 – 7. PMID: 26190673
- Reades R et al. Intraosseous Versus Intravenous Vascular Access During Out-of-Hospital Cardiac Arrest: A Randomized Controlled Trial. Ann Emerg Med 2011; 58 (6): 509 – 16. PMID: 21856044
- Link MS et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132 (18 Suppl 2): S444 – 64. PMID: 26472995
- Littmann et al. A Simplified and Structured Teaching Tool for the Evaluation and Management of Pulseless Electrical Activity. Med Princ Pract 2014; 23 (1): 1 – 6. PMID: 23949188
- Ochoa FJ et al. Competence of Health Professionals to Check the Carotid Pulse. Resuscitation 1998; 37: 173 – 75. PMID: 9715777
- Blyth L et al. Bedside Focused Echocardiography as Predictor of Survival in Cardiac Arrest Patients: A Systematic Review. Academic Emergency Medicine 2012; 19 (10): 1119 – 26. PMID: 23039118
- Ahrens T et al. End-tidal carbon dioxide measurements as a prognostic indicator of outcome in cardiac arrest. Am J Crit Care 2001; 10(6): 391 – 8. PMID: 11688606
- Paley J et al. Introsseous Infusion Rates Under High Pressure: A Cadaveric Comparison of Anatomic Sites. J Trauma Acute Care Surg 2015; 78 (2): 295 – 9. PMID: 25757113
- Benger JR et al. Effect of a Strategy of a Supraglottic Airway Device vs Tracheal Intubation During Out-of-Hospital Cardiac Arrest on Functional Outcome: The AIRWAYS-2 Randomized Clinical Trial. JAMA 2018. [Epub Ahead of Print]
- Jabre P et al. Effect of Bag-Mask Ventilation vs Endotracheal Intubation During Cardioplmonary Resuscitation on Neurological Outcome after Out-of-Hospital Cardiorespiratory Arrest: A Randomized Clinical Trial. JAMA 2018 [Epub Ahead of Print]
- Perkins GD et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. NEJM 2018. [Epub Ahead of Print]
- Bergum D et al. ECG Patterns in Early Pulseless Electrical Activity- Associations with Aetiology and Survival of In-Hospital Cardiac Arrest. Resuscitation 2016. PMID: 27143124
- Gardner, KF et al. The Cardiac Arrest Sonographic Assessment (CASA) Exam – A Standardized Approach to the use of Ultrasound in PEA. AJEM 2018. PMID: 28851499
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)



